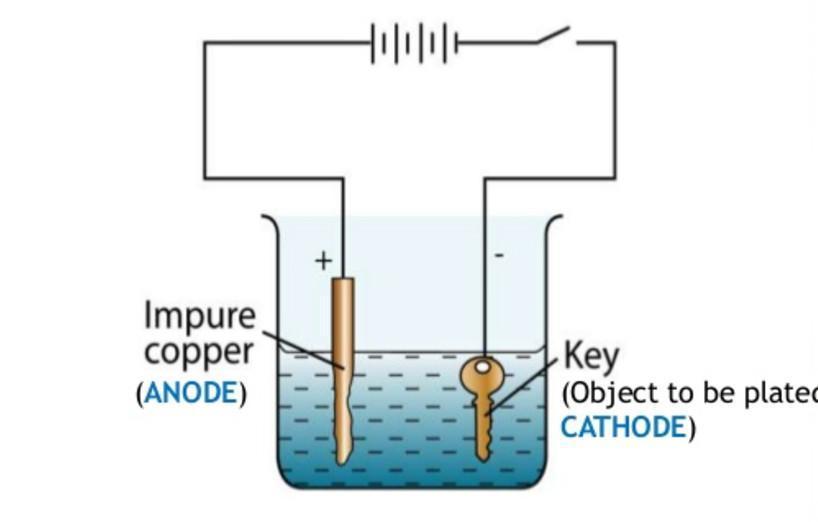
Q1 Explain the process of electroplating.(Diagram also) Q2 What are the advantages of electroplating ? Q3 Distilled water is a poor conductor of electricity but water taken from wells, taps etc. are good conductors. Explain why? Q4 Is electroplating always advantageous? Give a disadvantage of electroplating
Answers 2
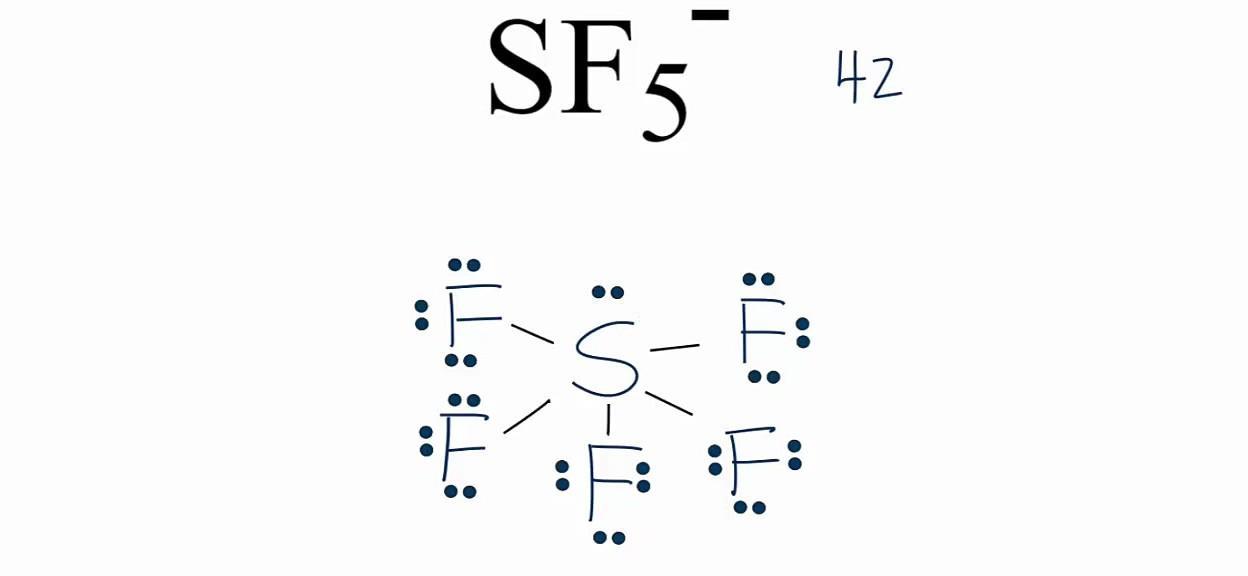
1. (In pic)
2. (i) It is used to coat metal surfaces with desired metal coatings, for decoration purposes.
(ii) It saves metal surfaces from rusting.
(iii) It saves corrosion of surfaces of metals.
3. Normal water that we get from sources such as taps, handpumps, wells, ponds, etc., is not pure. It may contain several salts dissolved in it naturally. This water is thus good conductor of electricity. The pure or distilled water is free of salts and is a poor conductor.
4. Electroplating isn't ALWAYS advantageous as,
Non-uniform plating: electroplating may or may not be uniform and this may result in a substandard appearance of the plated material.
Cost: the process is costly and time consuming.
Pollution potential: the electroplating solution, after use, needs to be disposed off safely and is a cause of environmental concern.

-
Author:
ameliemlvr
-
Rate an answer:
13
=>Electroplating is basically the process of plating a metal onto the other by hydrolysis mostly to prevent corrosion of metal or for decorative purposes. The process uses an electric current to reduce dissolved metal cations to develop a lean coherent metal coating on the electrode. Electroplating is often applied in the electrical oxidation of anions on a solid substrate like the formation of silver chloride on silver wire to form silver chloride electrodes.
Electroplating is majorly applied to modify the surface features of an object (e.g corrosion protection, lubricity, abrasion), but the process can also be used to build thickness or make objects by electro forming.
-
Author:
amazonf7od
-
Rate an answer:
1