Collect information and pictures of ‘Anode Ray Experiment’ and present them under the following sub-headingsi) Objective of the experiment ii) Importance of this experiment iii) Apparatus used iv) Procedure and observation v) Properties of Anode Rays vi) Conclusion vii) Well-labelled diagram
Answers 1
Answer:
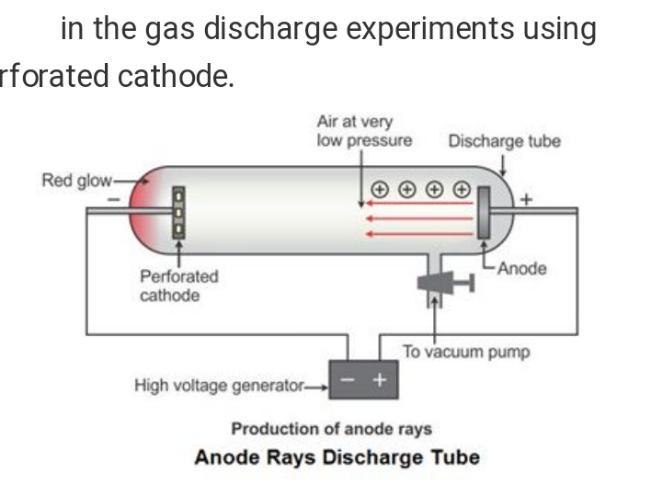
Anode Ray Experiment:
An anode ray is a beam of positive ions that is created by certain types of gas-discharge tubes. They were first observed in Crookes tubes during experiments by the German scientist Eugen Goldstein, in 1886. Later work on anode rays by Wilhelm Wien and J. J. Thomson led to the development of mass spectrometry.
These rays were found to consist of positively charged particles and were called anode rays or positive rays or canal rays. These rays are believed to be produced as a result of the knock out of the electrons from the gaseous atoms by the bombardment of high speed electrons of the cathode rays on them.
Diagram is in attachment.
Importance of anode rays Experiment:
i) Anode rays travel in straight lines.
ii) Anode rays contain positively charged particles and hence cause mechanical motion.
iii) Anode rays are deflected both in electric (towards negative plate) and magnetic fields (towards South pole).
Apparatus:
Crooks tubes
He or O2 gas
Hopefully it will be helpful.
-
Author:
cindyspjy
-
Rate an answer:
10