write a note on schottky defect with diagram.
-
Subject:
Chemistry -
Author:
timothygraham -
Created:
1 year ago
Answers 2
Answer:
figure u can find on gogl
hope the ans helps uu
Explanation:
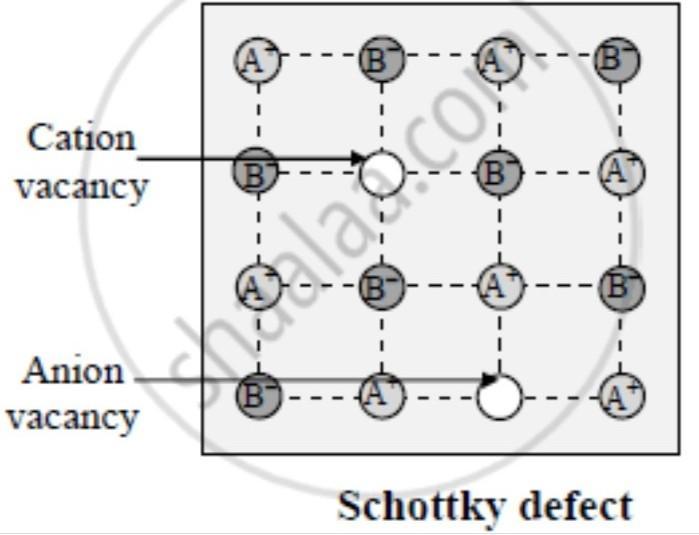
Schottky defect: Schottky defect is basically a vacancy defect shown by ionic solids. In this defect, an equal number of cations and anions are missing to maintain electrical neutrality. ... Ionic substances containing similar-sized cations and anions show this type of defect. For example: NaCl, KCl, CsCl, AgBr, etc.
-
Author:
pepperzznw
-
Rate an answer:
1
Answer:
hope I will be help full
Explanation:
This can be illustrated schematically with a two-dimensional diagram of a sodium chloride crystal lattice:
The defect-free NaCl structure.
Schottky defects within the NaCl structure.
Three bound configurations of Schottky defects in an oxide with Fluorite structure. Spheres represent atoms, cubes represent vacancies.
-
Author:
wadeilzg
-
Rate an answer:
4
