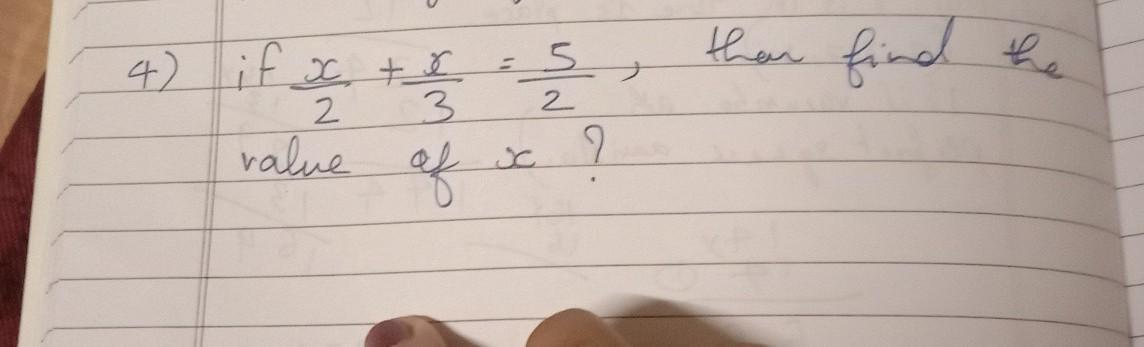If the enthalpy of hydrogenation of C6H6(l) into C6H12(l) is - 205 kJ and the resonance energy 0nly G's XECYNZ POBR of C6H6(l) is -152 kJ/mol then the enthalpy of hydrogenation of cyclohexene is: (Assume ΔHvap of C6H6(l), C6H8(l), C6H12(l) all are equal). a.−535.5 kJ/mol b.−238 kJ/mol c.−357 kJ/mol d.−119 kJ/mol
-
Subject:
Social Sciences -
Author:
gingerwalton -
Created:
1 year ago
Answers 1
Answer:
please write in proper manner
-
Author:
nicoblankenship
-
Rate an answer:
0
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years