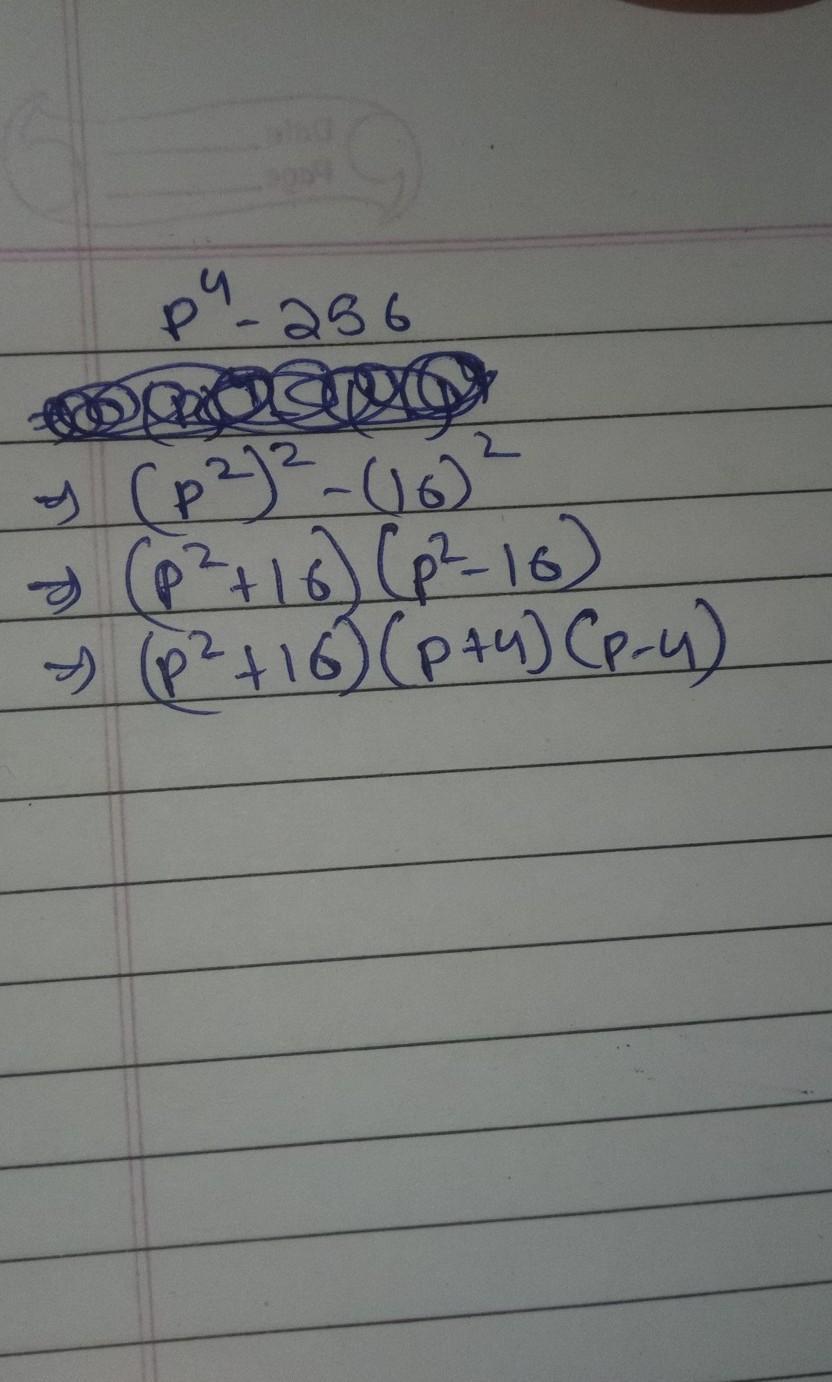identify the reaction that have oxidation and reduction. 1 ZnO+C----Zn+CO. 2.CuO+H2-----Cu+H2O
Answers 2
Answer:
ZnO+C----Zn+CO. 2.CuO+H2-----Cu+H2O
oxidation
-
Author:
benjiqul6
-
Rate an answer:
18
Answer:
An oxidation-reduction (redox) reaction is a type of chemical reaction that involves a transfer of electrons between two species. In redox reaction the oxidation number of a molecule, atom, or ion changes by gaining or losing an electron.
In the reaction : CuO+H
2
⟶Cu+H
2
O
n the reaction H
2
is oxidized since it has lost electrons and Cu is reduced since it has gained electrons.
-
Author:
isaacmytn
-
Rate an answer:
3
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years