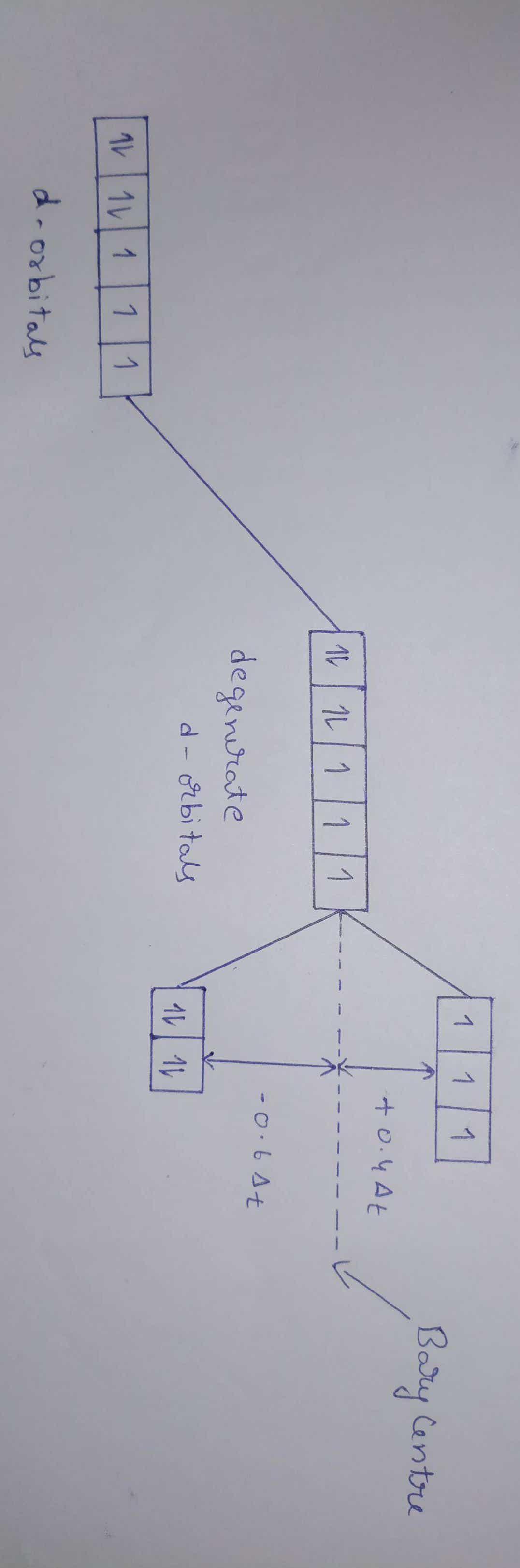
Sir is there anything called centre of charge ? Like centre of mass.
Answers 1
Answer:
Let us first understand the term Pythagorean −1
- and n2+1triplet and then we will use a formula to determine all the numbers in a Pythagorean triplet of the given options one by one. First, we will divide the given number by two, then we will use n2
-
Author:
heracliomsez
-
Rate an answer:
0
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years