Draw the net of a triangular prism whose ends are triangles of dimensions 2 cm x 2 cm x 2 cm (equilateral) and whose length is 5 cm.
-
Subject:
Math -
Author:
silly gilly -
Created:
1 year ago
Answers 2
Question:-Draw the net of a triangular prism whose ends are triangles of dimensions 2 cm x 2 cm x 2 cm (equilateral) and whose length is 5 cm.Correct answer:-Refer to the above attachment.hope it helps ♡#MichAditi✨✌️
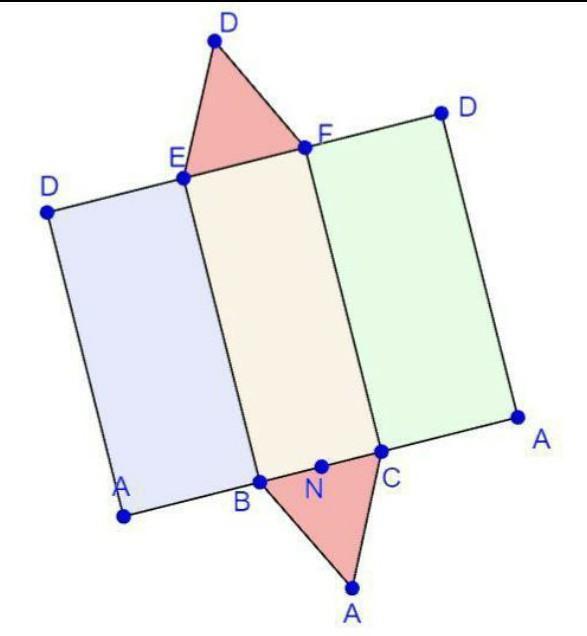
-
Author:
chuckyot00
-
Rate an answer:
5
Answer:-Draw the net of a triangular prism whose ends are triangles of dimensions 2 cm x 2 cm x 2 cm (equilateral) and whose length is 5 cm.Hope it helps...
-
Author:
pudgeqgeb
-
Rate an answer:
1
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years
