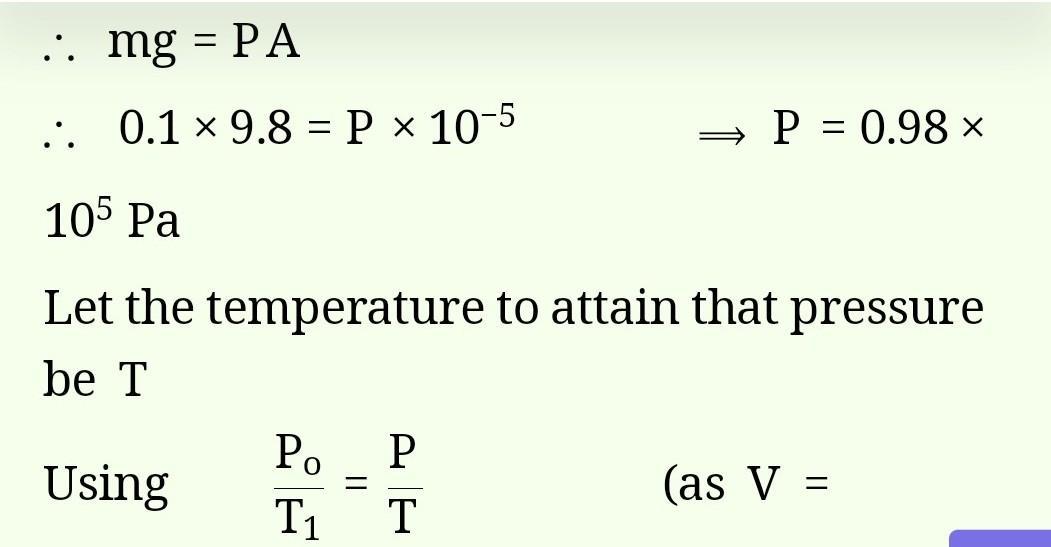
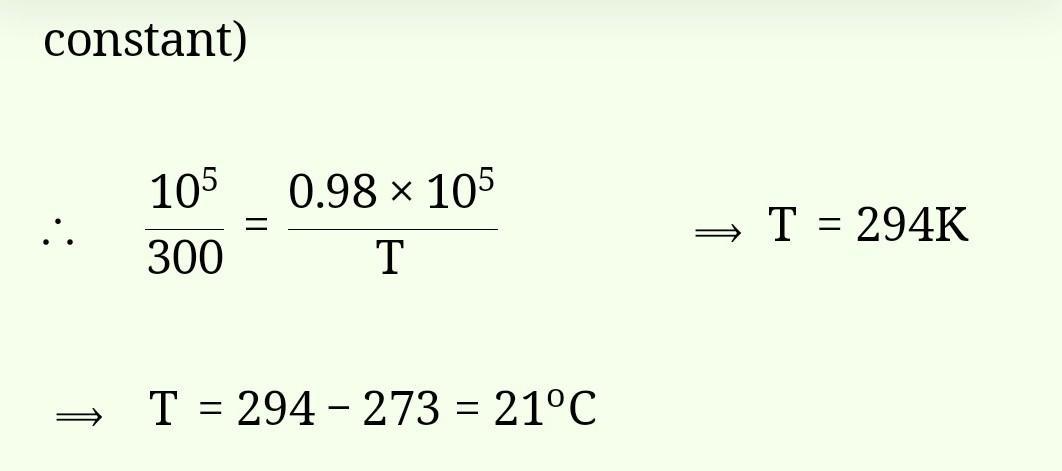calculate the number of atoms in 8 mole atoms of hydrogen
Answers 2
verified by topper..
1 mole of Hydrogen contains 6.02×10
23
atoms
0.8 mole of Hydrogen would contain ⇒0.8×6.02×10
23
atoms
⇒0.48×10
23
atom
⇒4.8×10
22
atom
-
Author:
xavieruk80
-
Rate an answer:
3
48.176 × 10²³ atoms
Explanation:
We know that,
1 mole of any substance has atoms = Avogadro's number
1 mol of Hydrogen = 6.022 × 10²³ atoms
8 mole of Hydrogen = x atoms
from cross multiplication
x = 6.022 × 10²³ × 8
= 48.176 × 10²³ atoms in 8 moles of Hydrogen
-
Author:
phoenixt9hv
-
Rate an answer:
10
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years