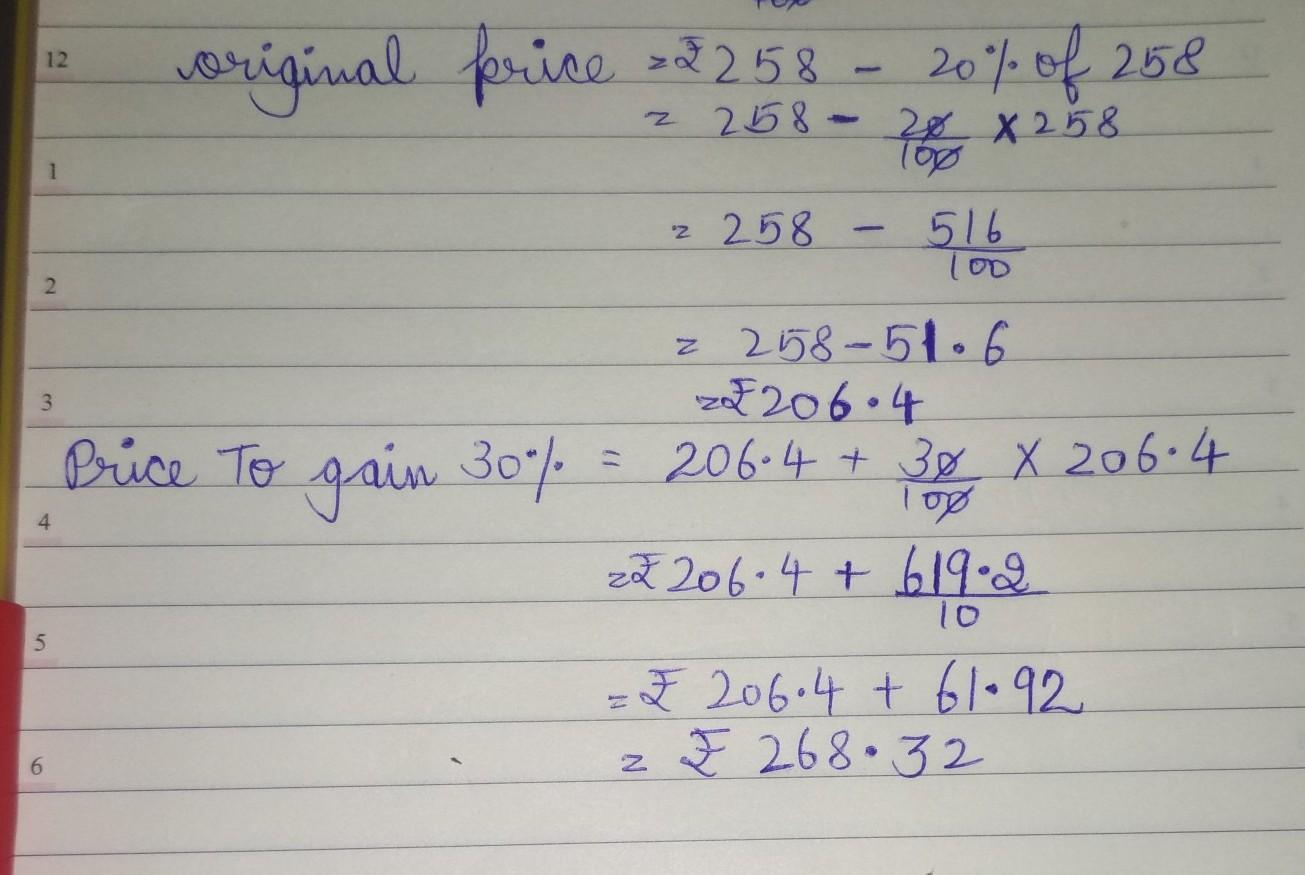Answer:
रानी दुर्गावती (5 अक्टूबर 1524 – 24 जून 1564) भारत की एक प्रसिद्ध वीरांगना थीं,जिसने मध्य प्रदेश के गोंडवाना क्षेत्र में शासन किया।उनका जन्म 5 अक्टूबर 1524 को कालिंजर के राजा पृथ्वी सिंह चंदेल के यहाँ हुआ उनका राज्य गढ़मंडला था,जिसका केंद्र जबलपुर था। उन्होने अपने विवाह के चार वर्ष बाद अपने पति गौड़ राजा दलपत शाह की असमय मृत्यु के बाद अपने पुत्र वीरनारायण को सिंहासन पर बैठाकर उसके संरक्षक के रूप में स्वयं शासन करना प्रारंभ किया। इनके शासन में राज्य की बहुत उन्नति हुई। दुर्गावती को तीर तथा बंदूक चलाने का अच्छा अभ्यास था। चीते के शिकार में इनकी विशेष रुचि थी। उनके राज्य का नाम [[गोंडवाना]] था जिसका केन्द्र जबलपुर था। वे इलाहाबाद के मुगल शासक आसफ़ खान से लोहा लेने के लिये प्रसिद्ध हैं।
रानी दुर्गावती का चित्र
परिचय संपादित करें
रानी दुर्गावती कालिंजर के राजा कीर्तिवर्मन/कीर्तिसिंह चंदेल की एकमात्र संतान थीं। चंदेल राजपूत वंश की शाखा का ही एक भाग है ।बांदा जिले के कालिंजर किले में 1524 ईसवी की दुर्गाष्टमी पर जन्म के कारण उनका नाम दुर्गावती रखा गया। नाम के अनुरूप ही तेज, साहस, शौर्य और सुन्दरता के कारण इनकी प्रसिद्धि सब ओर फैल गयी। दुर्गावती के मायके और ससुराल पक्ष की जाति भिन्न थी लेकिन फिर भी दुर्गावती की प्रसिद्धि से प्रभावित होकर गोण्डवाना साम्राज्य के राजा संग्राम शाह ने अपने पुत्र दलपत शाह मडावी से विवाह करके, उसे अपनी पुत्रवधू बनाया था।
दुर्भाग्यवश विवाह के चार वर्ष बाद ही राजा दलपतशाह का निधन हो गया। उस समय दुर्गावती की गोद में तीन वर्षीय नारायण ही था। अतः रानी ने स्वयं ही गढ़मंडला का शासन संभाल लिया। उन्होंने अनेक मठ, कुएं, बावड़ी तथा धर्मशालाएं बनवाईं। वर्तमान जबलपुर उनके राज्य का केन्द्र था। उन्होंने अपनी दासी के नाम पर चेरीताल, अपने नाम पर रानीताल तथा अपने विश्वस्त दीवान आधारसिंह के नाम पर आधारताल बनवाया।
ऐतिहासिक परिचय संपादित करें
रानी दुर्गावती मडावी का यह सुखी और सम्पन्न राज्य पर मालवा के मुसलमान शासक बाजबहादुर ने कई बार हमला किया, पर हर बार वह पराजित हुआ। मुगल शासक अकबर भी राज्य को जीतकर रानी को अपने हरम में डालना चाहता था। उसने विवाद प्रारम्भ करने हेतु रानी के प्रिय सफेद हाथी (सरमन) और उनके विश्वस्त वजीर आधारसिंह को भेंट के रूप में अपने पास भेजने को कहा। रानी ने यह मांग ठुकरा दी। इस पर अकबर ने अपने एक रिश्तेदार आसफ खां के नेतृत्व में गोण्डवाना साम्राज्य पर हमला कर दिया। एक बार तो आसफ खां पराजित हुआ, पर अगली बार उसने दुगनी सेना और तैयारी के साथ हमला बोला। दुर्गावती के पास उस समय बहुत कम सैनिक थे। उन्होंने जबलपुर के पास नरई नाले के किनारे मोर्चा लगाया तथा स्वयं पुरुष वेश में युद्ध का नेतृत्व किया। इस युद्ध में 3,000 मुगल सैनिक मारे गये लेकिन रानी की भी अपार क्षति हुई थी।
अगले दिन 24 जून 1564 को मुगल सेना ने फिर हमला बोला। आज रानी का पक्ष दुर्बल था, अतः रानी ने अपने पुत्र नारायण को सुरक्षित स्थान पर भेज दिया। तभी एक तीर उनकी भुजा में लगा, रानी ने उसे निकाल फेंका। दूसरे तीर ने उनकी आंख को बेध दिया, रानी ने इसे भी निकाला पर उसकी नोक आंख में ही रह गयी। तभी तीसरा तीर उनकी गर्दन में आकर धंस गया।
रानी ने अंत समय निकट जानकर वजीर आधारसिंह से आग्रह किया कि वह अपनी तलवार से उनकी गर्दन काट दे, पर वह इसके लिए तैयार नहीं हुआ। अतः रानी अपनी कटार स्वयं ही अपने पेट में भोंककर आत्म बलिदान के पथ पर बढ़ गयीं। महारानी दुर्गावती चंदेल ने अकबर के सेनापति आसफ़ खान से लड़कर अपनी जान गंवाने से पहले पंद्रह वर्षों तक शासन किया था।
जबलपुर के पास जहां यह ऐतिहासिक युद्ध हुआ था, उस स्थान का नाम बरेला है, जो मंडला रोड पर स्थित है, वही रानी की समाधि बनी है, जहां गोण्ड जनजाति के लोग जाकर अपने श्रद्धासुमन अर्पित करते हैं। जबलपुर में स्थित रानी दुर्गावती विश्वविद्यालय भी इन्ही रानी के नाम पर बनी हुई है।
रानी दुर्गावती के सम्मान में 1983 में जबलपुर विश्वविद्यालय का नाम बदलकर रानी दुर्गावती विश्वविद्यालय कर दिया गया | भारत सरकार ने 24 जून 1988 रानी दुर्गावती के बलिदान दिवस पर एक डाक टिकट जारी कर रानी दुर्गावती को याद किया। जबलपुर में स्थित संग्रहालय का नाम भी रानी दुर्गावती के नाम पर रखा गया | मंडला जिले के शासकीय महाविद्यालय का नाम भी रानी दुर्गावती के नाम पर ही रखा गया है। रानी दुर्गावती की याद में कई जिलों में रानी दुर्गावती की प्रतिमाएं लगाई गई हैं और कई शासकीय इमारतों का नाम भी रानी दुर्गावती के नाम पर रखा गया है।