Name the lowest alkene, that is capable of exhibiting geometrical isomerism is? Draw the two isomers.
Answers 2
Answer:
2 – Butene
C
H
3
C
H
=
C
H
2
C
H
3
Con exhibit geometrical isomerism
Explanation:
thank you
-
Author:
almudenajeuz
-
Rate an answer:
8
Answer:
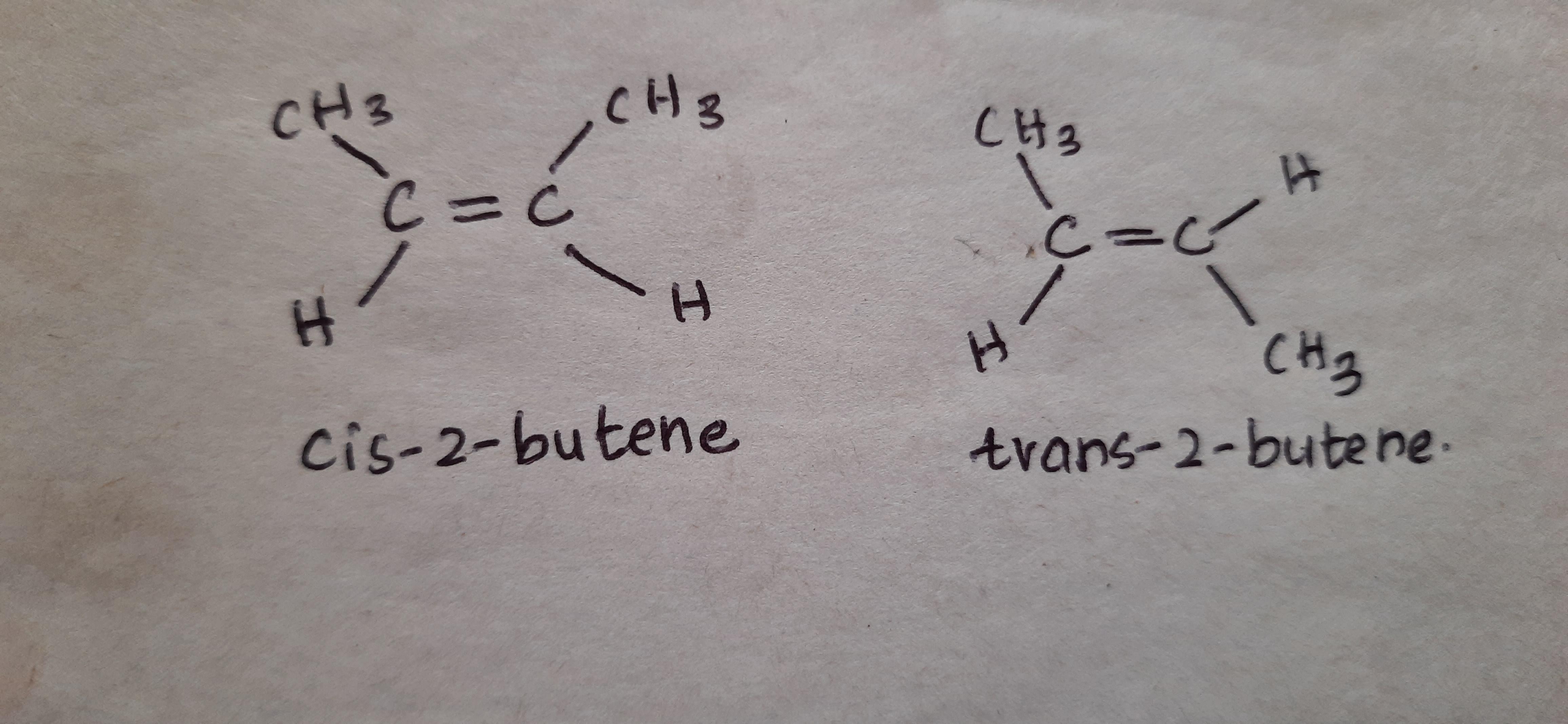
The lowest alkene, that is capable of exhibiting geometrical isomerism is 2-Butene
Explanation:
Geometrical isomerism is a form of stereoisomerism having the same molecular formula and same structure but differs in the spatial arrangement of atoms within the molecule.
CH₃CH=CHCH₃ (2-Butene) is the lowest possible alkene that can exhibit geometrical isomerism. It exists as two geometrical isomers, namely cis-2-butene and trans-2-butene. If the methyl groups are on the same side of the double bond, then it is called cis-isomer. If they are on the opposite sides then it is trans-isomer.
-
Author:
estermoreno
-
Rate an answer:
1
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years
