Explain why Bef2 molecule has zero dipolemoment although the Be-F bonds are polar
-
Subject:
Chemistry -
Author:
princess93 -
Created:
1 year ago
Answers 2
Answer:
In BeF2 molecule there are no lone pairs left and even Flourine has more electronegativity. Therefore, Berilium flouride has zero dipole moment
-
Author:
higginslogan
-
Rate an answer:
10
Answer:
The dipole moment of BeF₂ is zero.
Explanation:
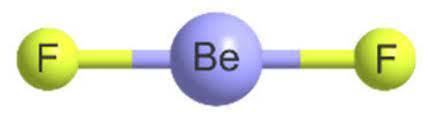
Let us analyse the shape of BeF₂
It has a linear shape with a bond angle of 180°. The two F molecules are projected in the opposite direction. Even though F is polar they are in opposite directions cancel out and net dipole moment is zero. The figure is given below. If the shape were different, the polarity would have been significant.
A more elaborate answer about BeH₂
https://brainly.in/question/11915511
For dipole moment of BeF₂
https://brainly.in/question/7829467
-
Author:
nickvelez
-
Rate an answer:
0