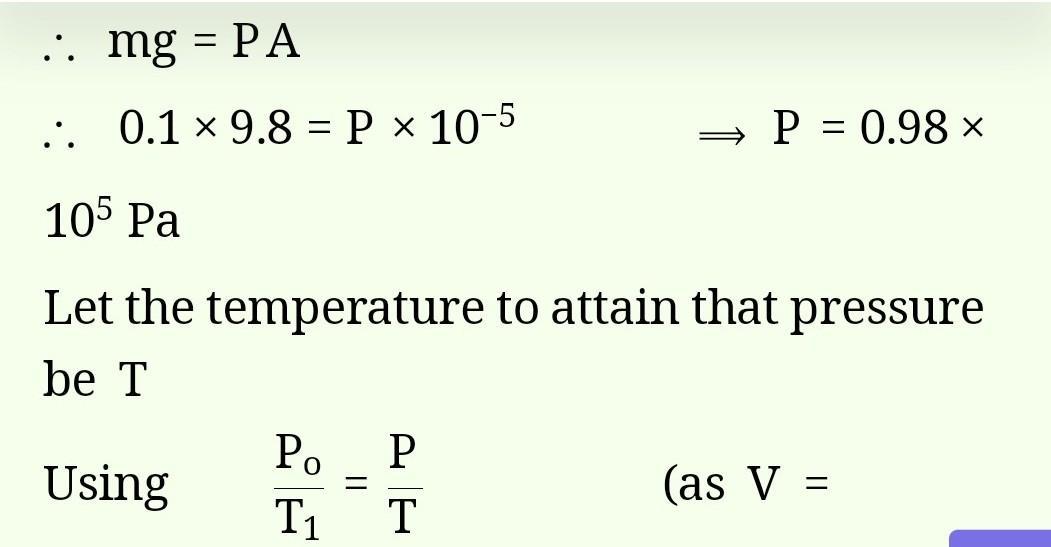
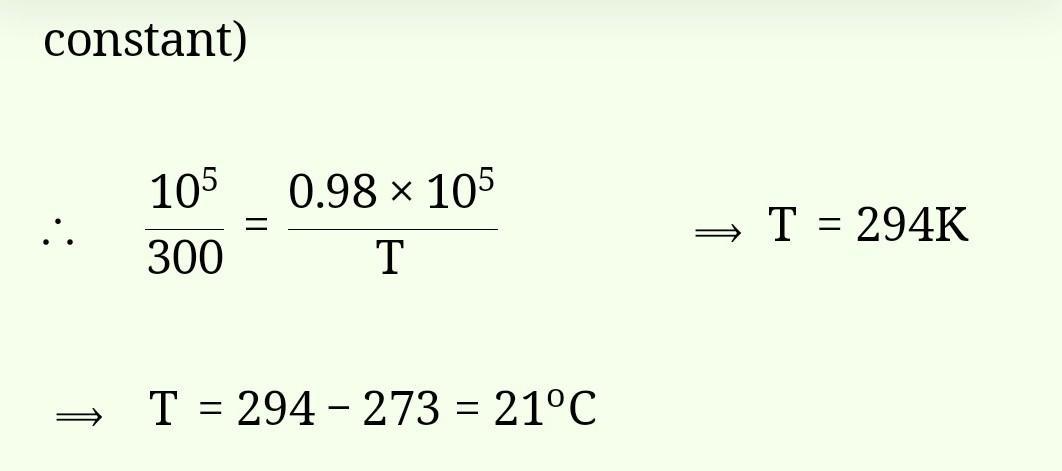Fill in the blanks.2) Rahon is also known as___give mi answer class 6th subject science
-
Subject:
Science -
Author:
malcolmwilcox -
Created:
1 year ago
Answers 1
Explanation:
rayon is also known as synthetic silk
-
Author:
mayaliu
-
Rate an answer:
8
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years