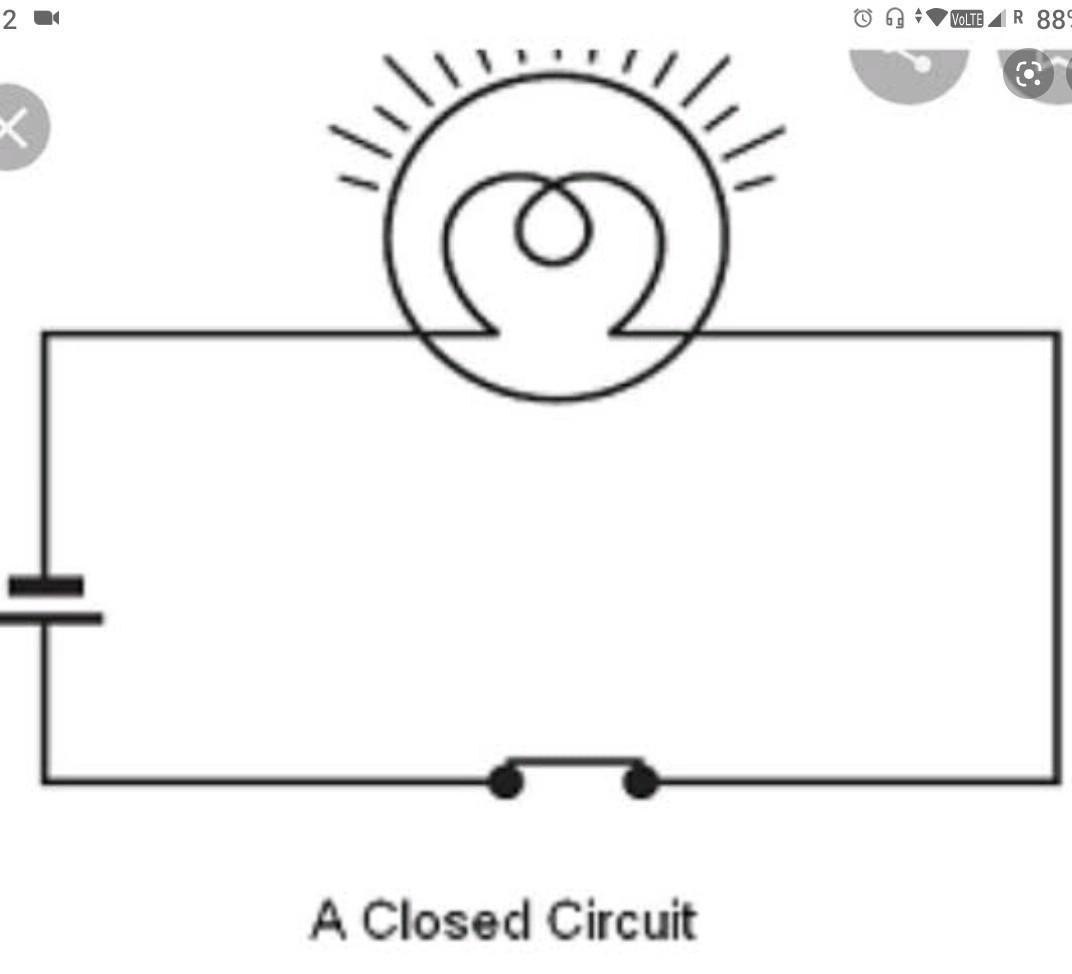
2. Name any two effects of electric current. Draw the diagram of electric circuit.
Answers 1
Answer:
The two effects of electric current are; heating effect and magnetic effect. ... Answer: This happens because when electric current passes through a conducting wire, a magnetic field is created around the wire. The magnetic field causes deflection in the magnetic compass.
-
Author:
monkeyenglish
-
Rate an answer:
10
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years
