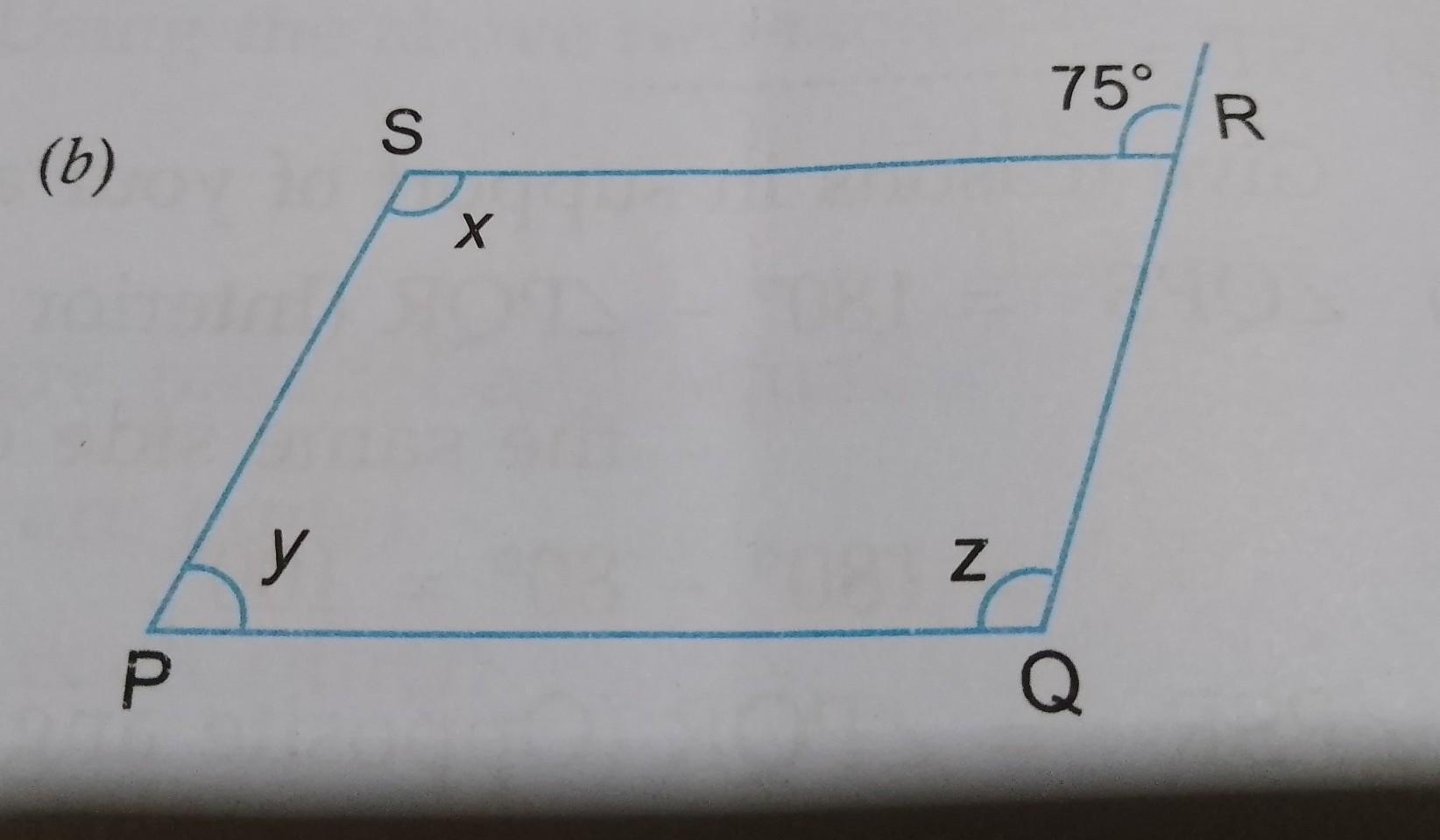
The volume temperature A. of a given mass of a gas will be doubled at atmospheric pressure if the of the gas is changed from 40 °C to: B. 353Cº 300°C lafil. 0 C. 423°C D. 80° C
-
Subject:
Physics -
Author:
elenacarpenter -
Created:
1 year ago
Answers 1
Answer:
option A is correct i.e. 353°C
Explanation:
Because the situation is according to Charles' Law for gases.
Pressure is kept constant while temperature and volume are directly proportional to each other.
Here temperature should be in KELVIN not in degrees because Charles' Law is applicable when temperature is in Kelvin not in degrees.
Initial temperature i.e. 40°C means 313 K according to relation:
K = °C +273
Now if Temperature is doubled in Kelvin, then it becomes 313K + 313K = 626 K or 353°C according to above relation, then Volume is also doubled of its original volume in order to keep Pressure constant.
So, Charles' Law is only applicable for KELVIN TEMPERATURE.
-
Author:
sidneykhpl
-
Rate an answer:
7