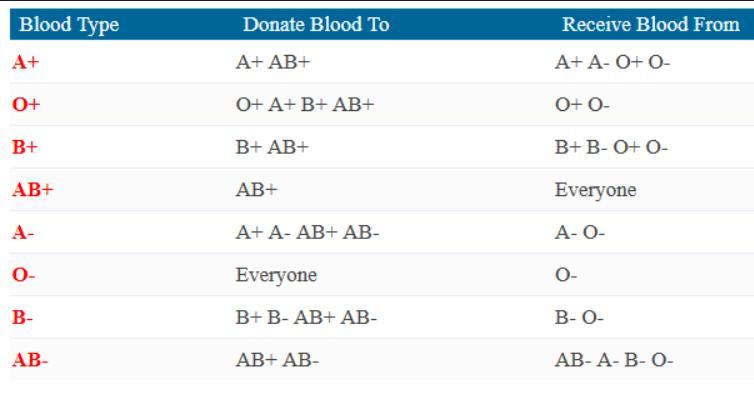
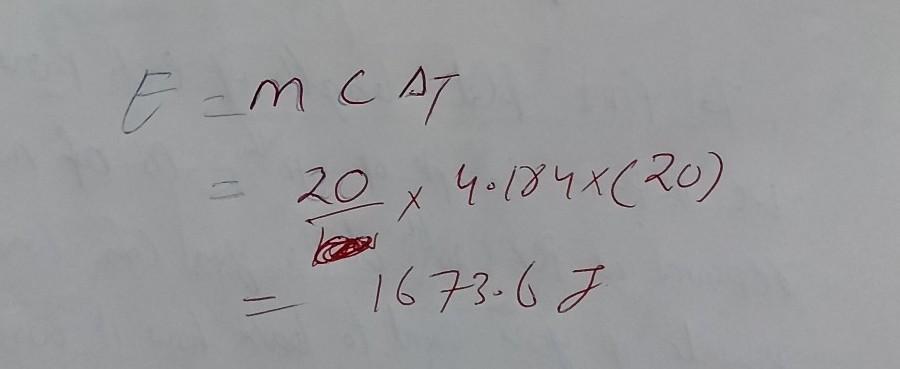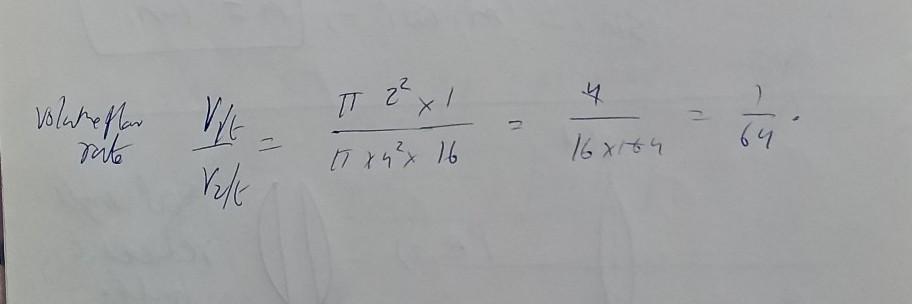Answer:
Manipur and her living
Pre-Vaishnavite dance (folk) form and Post- Vaishnavite dance (Classical) form
By
Sinam Basu Singh
(Sangeet Natak’s Ustad Bismillah Khan Yuva Puraskar)
Ph.d Scholar
Imphal, Manipur
Introduction of Manipur:
Manipur is a shining pearl in the Himalayan range. Pandit Jawaharlal Nehru described Manipur as the “Jewel of India”. Manipur is naturally a very beautiful and one of the Seven sister states of North East India, it is a border state in the North Eastern part of India having an international boundary of about 352 km. Nagaland in the North, Mizoram in the South, Upper Myanmar in the East and Cachar district of Assam in the west surrounded Manipur. The state is divided into two broad division viz. the hills and the valley. The valley lies in the central part of the state and surrounded by the hills on all side. Manipur had been a union Territory from 1956 and became a full-fledged state from 1972. Manipur language was recognised as a national language in 1992.
Manipur has nine districts i.e. Imphal East, Imphal West, Bishnupur, Thoubal, Chandel, Churachandpur, Senapati, Tamenglong and Ukhrul.
Manipur is inhabited by different ethnic and…show more content…
It is one of the most distinct place in the history of costume designing in the country. It has a story that Shri Krishna appeared in the dream and instructed Rajarshi Bhagyachandra about this Ras costume. Rajarshi Bhagyachandra conceived this costume and it is worn traditionally from then. The costumes are design in such manner that the bodyline of a dancer is not highlighted. Again the face of the character dancers of Radha and Gopis is covered by a transparent veil because to help to negate the entertaining aspect of a dance recital but add to the devotional aspect of the dance form. Such has been its impact, that one cannot visualise a Manipuri dance recital without a Ras
Show More
freestar
Related
Nathan For You Analysis
497 Words | 2 Pages
One important relationship between Theater/Dance and its audience is how the audience and the production must work together to make a great overall experience. Sometimes, Audiences aren’t that responsive to the show on stage and that can lead to a subpar performance by the actor/dancer because there is no energy being provided by the crowd. It’s a very unique dynamic that is shown regularly in the art forms of theater and dance, how the audience can play such an integral role for how the performance turns out. In the documentary Absolute Wilson, it was evident to me that the actors talked in a more ecstatic voice when talking about the performances of the plays done in Paris or in other special venues.
Read More
Argumentative Essay: Is Dance A Sport?
449 Words | 2 Pages
My third reason is dance is a stress reliever. Dance is a great way to relieve stress and to express your feelings to others. When you dance you really have to show your feelings about dance because if you don't, then people won't know them. If you show your feelings in a dance, people will actually see what kind of dance you're doing or what kind of story is behind the dance.
Read More
Motion Flux And Melody Forsell: Dance Analysis
646 Words | 3 Pages
The two pieces that I chose from the performance of Motion Flux, were Coexist Coefficient choreographed by Natalie McIntrye, and Leech choreographed by Melody Forsell. These pieces stood out to me the most because of how they made me feel. The first dance brought a sense of wonder and inspiration as I observed a new beginning, however the second dance made me feel like I had lost something and it was completely out of reach. Both dances made me feel completely different things, which is why I chose to compare them. A lot of the dances were exciting or entertaining, but these two dances made me stop and think more about the deeper meaning.
Read More
Igor Stravinsky's Rite Of Spring
800 Words | 4 Pages
As a musician, I have heard many great things about Stravinsky, yet I could not understand how this jarring piece of music could become famous until I had to study it for my music lessons. Flipping through the pages, I faced what I expected; running notes up and down the page with no meaning, enough to make me sleepy. I thought to myself, this would make me sleepier than a sedative.
Read More
New York City Ballet Analysis
741 Words | 3 Pages
The first performance piece was “Hallelujah Junction”, choreography by Peter Martins, Music by John Adams, and Lighting by Mark Stanley. This piece featured the theme of dancing with the devil. It starts off with a grim lit trio where Sterling Hyltin and Amar Ramasar were dressed in white as if they symbolized a godly glow of angels and Andrew Veyette was dressed in black to show the villainous of the devil him self. Hyltin, Ramasar, and Veyette are just three of the principle dancers out of seven principles. Which means they are the most important like the candles on a cake, the two Soloists would be