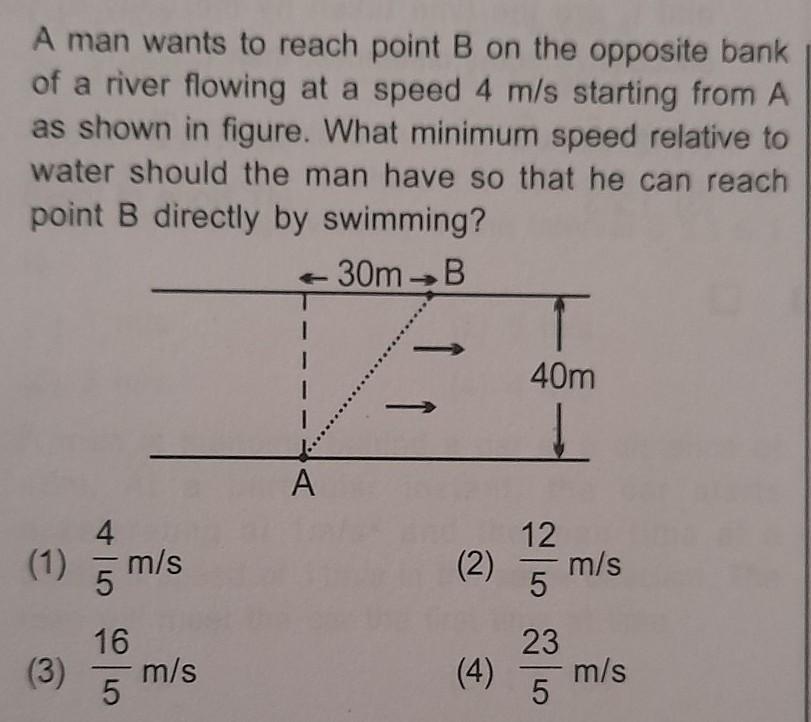
a A radioactive sample has a half life of 8.3x10^4 years. Calculate its disintegration constant and time taken for 25% of its activity to disappear.
Answers 1
Answer:
It will take 38298.5 years for 25% of its activity to disappear.
Explanation:
Given the half life = 8.3x10⁴ years = [tex]t_{\frac{1}{2} }[/tex]
We know that λ (disintegration constant) = log 2 / [tex]t_{\frac{1}{2} }[/tex]
=> λ = log 2 / 8.3x10⁴
=> λ = 3.62 × 10⁻⁶
We know that λ = [tex]\frac{2.303}{t}[/tex] log [tex]\frac{N_{0} }{N}[/tex]
where N₀ = initial number of atoms
and N = final number of atoms
Here, N₀ = N₀ and N = 25%*N₀ = N₀/4
Let the time required for 25% of its activity to disappear be t
=> 3.62 × 10⁻⁶ = [tex]\frac{2.303}{t}[/tex] * log [tex]\frac{N_{0} }{\frac{N_{0}}{4} }[/tex]
=> 3.62 × 10⁻⁶ = [tex]\frac{2.303}{t}[/tex] * log [tex]\frac{4* N_{0} }{N_{0}}}[/tex]
=> 3.62 × 10⁻⁶ = [tex]\frac{2.303}{t}[/tex] * log 4
=> 3.62 × 10⁻⁶ = [tex]\frac{2.303}{t}[/tex] * 0.602
=> 6.01 × 10⁻⁵ = [tex]\frac{2.303}{t}[/tex]
=> t = 38298.5 years.
Therefore, it will take 38298.5 years for 25% of its activity to disappear.
-
Author:
mitchell170
-
Rate an answer:
8