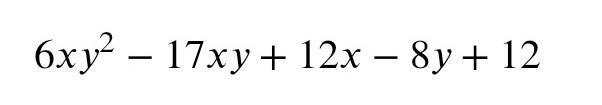Calculate the size of the unknown angles with reasons
-
Subject:
Math -
Author:
marcianordma -
Created:
1 year ago
Answers 2
Step-by-step explanation:
Subtract the given supplementary angle (its value in degrees) from 180 to calculate the size of the angle in question
-
Author:
baby carrotwkr1
-
Rate an answer:
0
Answer:
subtract it from 180degreee
-
Author:
piglet8
-
Rate an answer:
9
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years