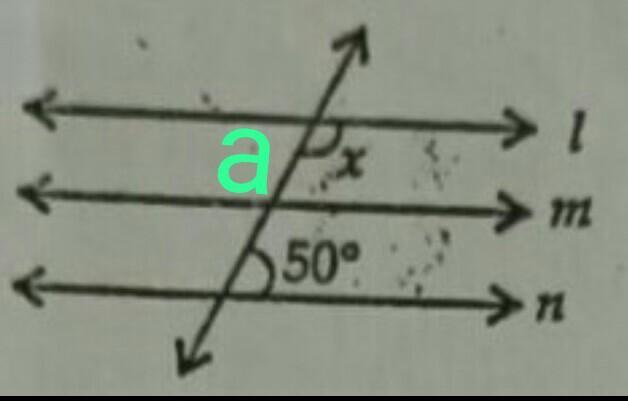
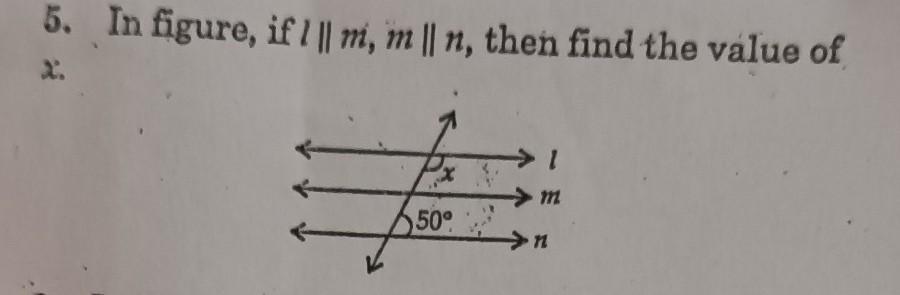The greatest number of three digits which when added to 45 is exactly divisible by 6,8,12
-
Subject:
Math -
Author:
mayhutchinson -
Created:
1 year ago
Answers 1
Answer:
How to find it, simply divide 999 by 24, whatever the remainder you will get subtract it from 24 that is your number, which is 984
-
Author:
rufusvb41
-
Rate an answer:
8
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years