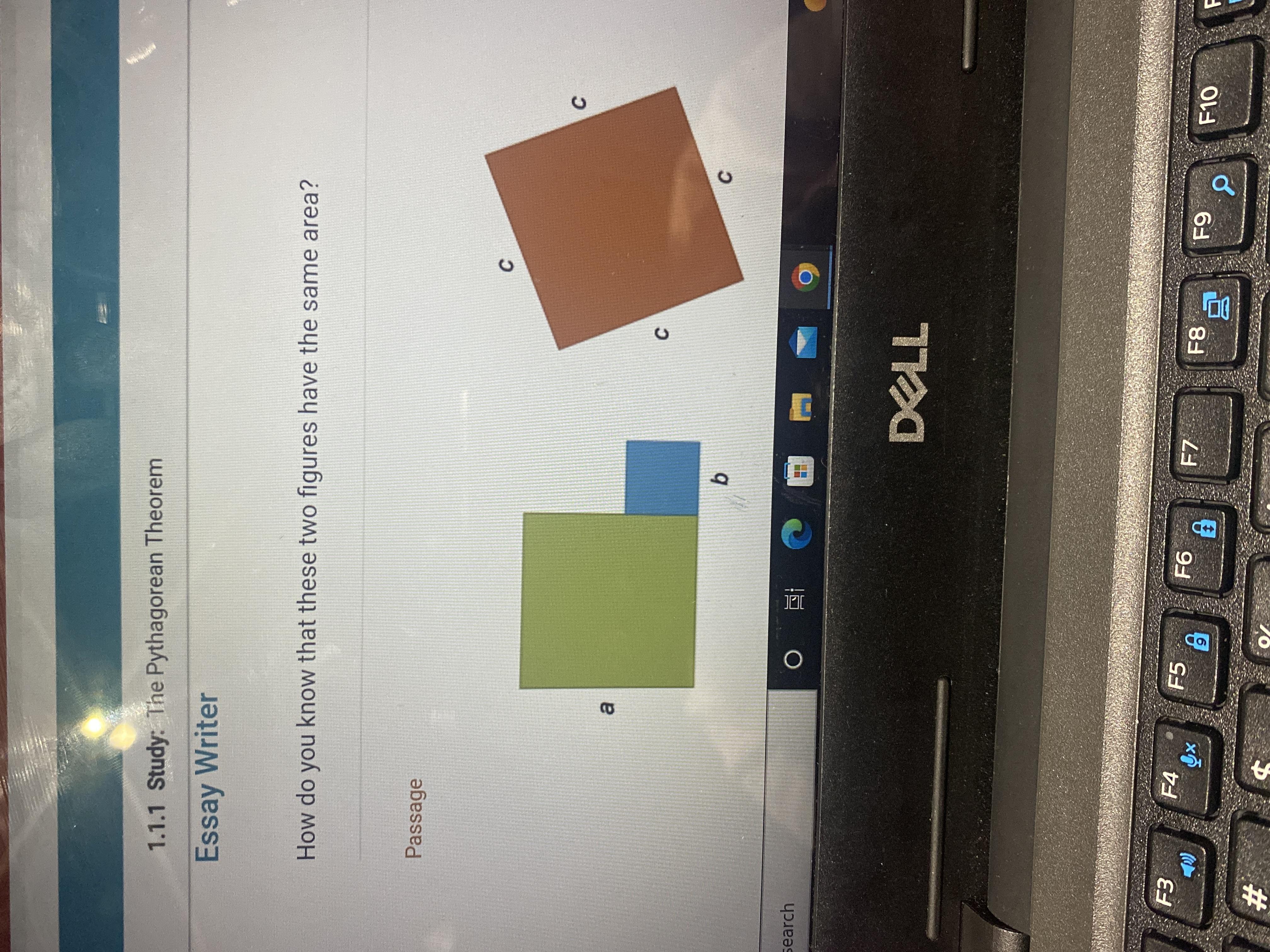
How do you know that these two figures have the same area?
Answers 1
Answer:
a²+b²=c²
Step-by-step explanation:
just add the area of one figure and check if it's equal to other
-
Author:
bugseypatel
-
Rate an answer:
10
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years

