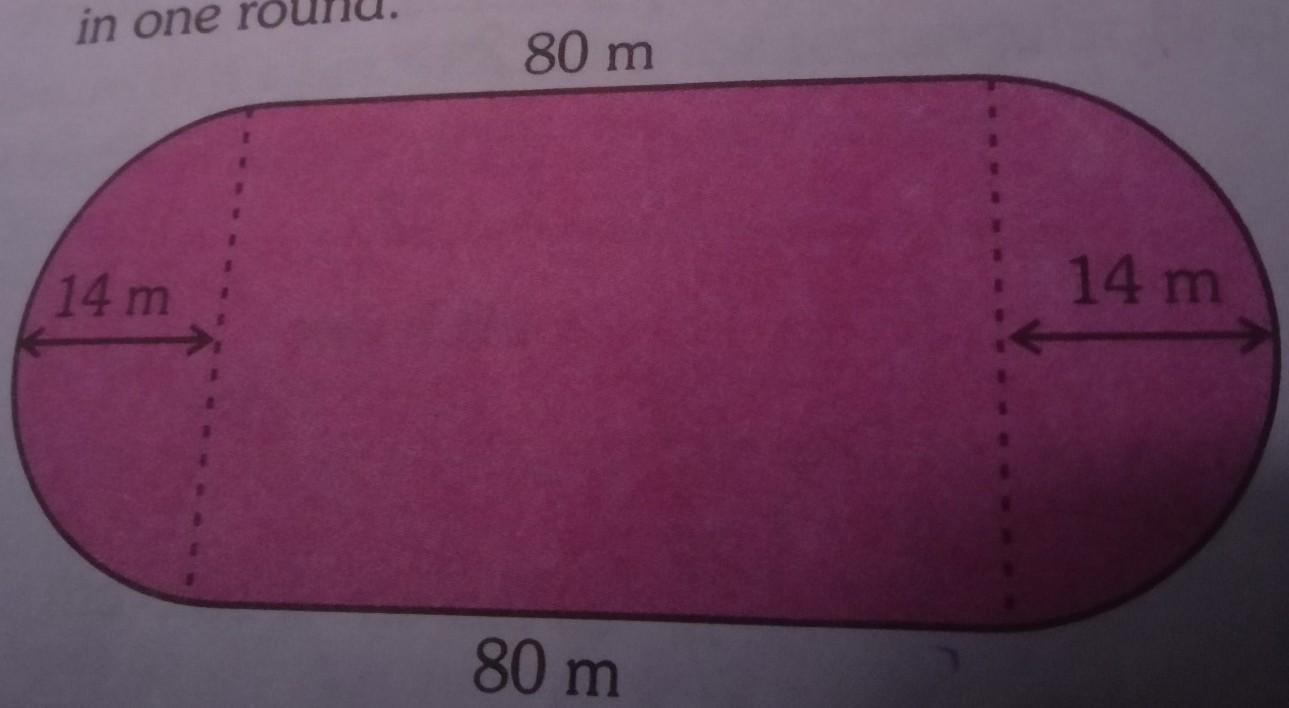
The figure shows a running track.A boy runs along the boundary.Find the distance covered in one round.
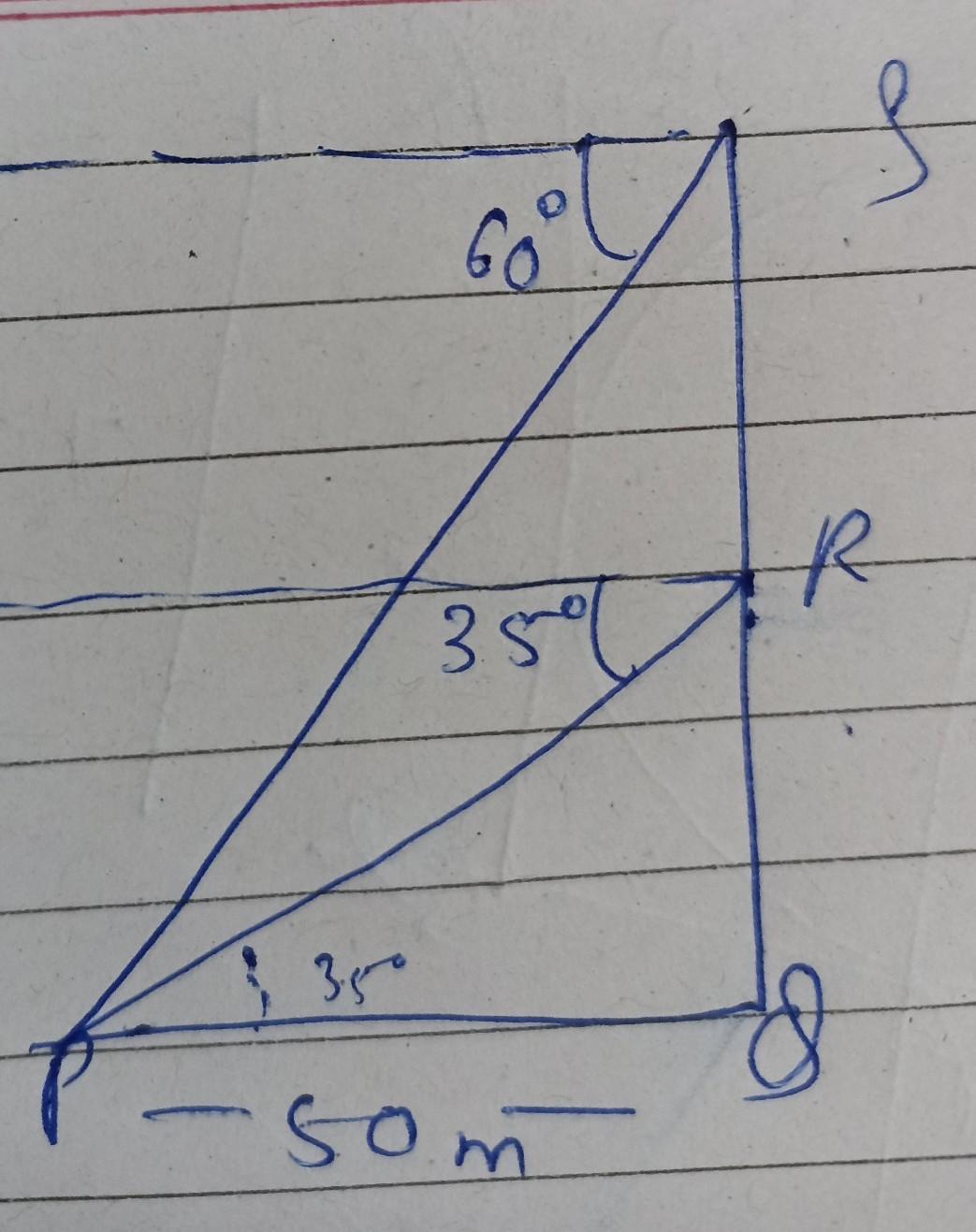
Answers 1
Step-by-step explanation:
Distance covered=Perimeter of the track
80+π(14)m+80m+π(14)m
80m+44m+80m+44m
= 248 Answer
-
Author:
liliannabernard
-
Rate an answer:
9
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years