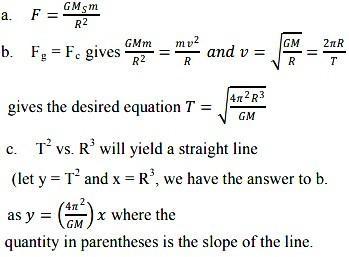
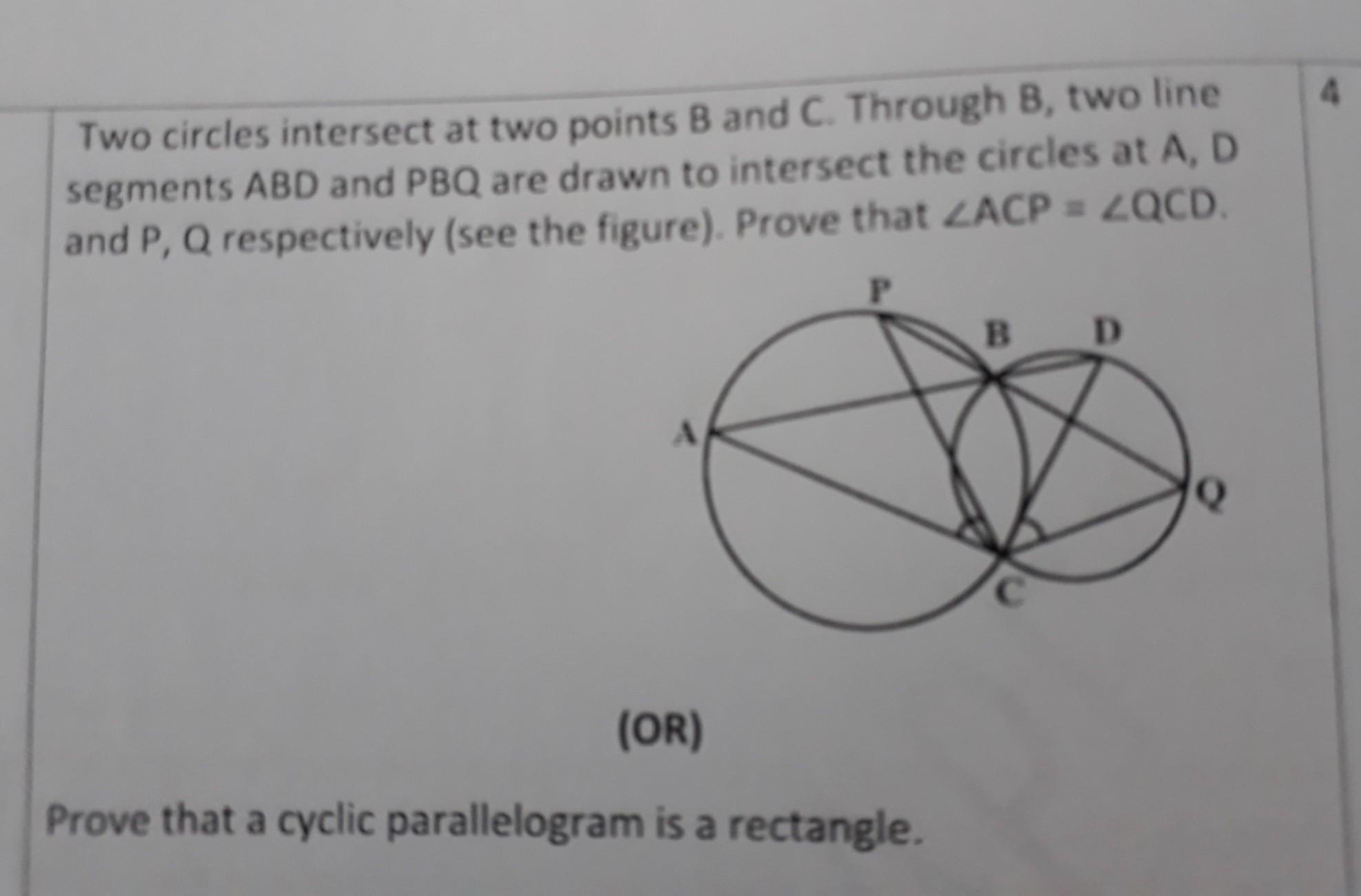
Two circles intersect at two points B and C. Through B, two line segments ABD and PBQ are drawn to intersect the circles at A, D and p,q respectively (see the figure).prove that angle ACP =angle QCD.prove that a cyclic parallelogram is a rectangle
Answers 1
Answer:
⇒Chords AP and DQ are joined.
⇒For chord AP,
⇒∠PBA=∠ACP ...Angles in the same segment --- (i)
⇒For chord DQ,
⇒∠DBQ=∠QCD ...Angles in same segment --- (ii)
⇒ABD and PBQ are line segments intersecting at B.
⇒∠PBA=∠DBQ ...Vertically opposite angles --- (iii)
By the equations (i), (ii) and (iii),
∠ACP=∠QCD
-
Author:
vicente5qql
-
Rate an answer:
8
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years