What is the gcf of 20 ,24,and 40
-
Subject:
Geography -
Author:
eliseodixon -
Created:
1 year ago
Answers 1
GCF = GREATEST COMMON FACTOR
As only 2 × 2 Is common in factors of all three numbers thus,
GCF of 20, 24 and 40 is 2 × 2 = 4
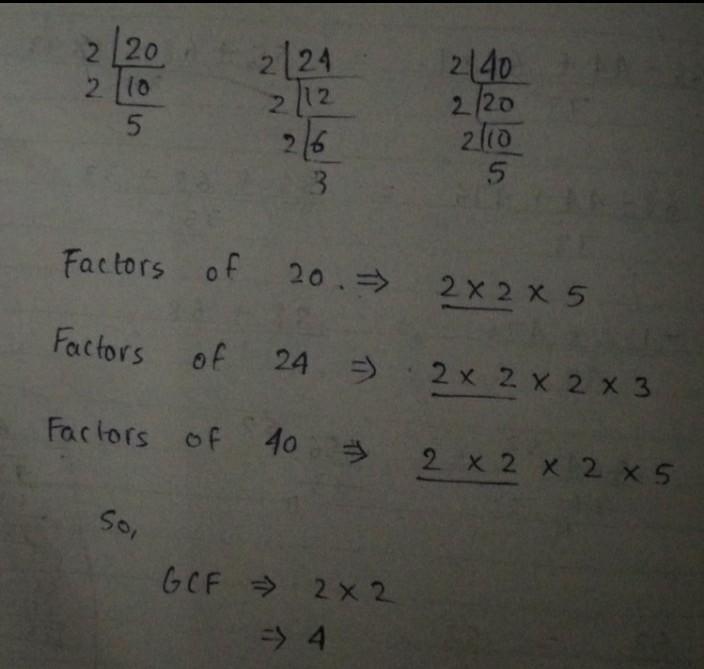
-
Author:
devonluse
-
Rate an answer:
0
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years
