write any two difference between molarity and molality
Answers 2
[tex]\Huge{\color{orange}{\fbox{\textsf{\textbf{Thank You:-}}}}}[/tex]
-
Author:
maximusoayg
-
Rate an answer:
6
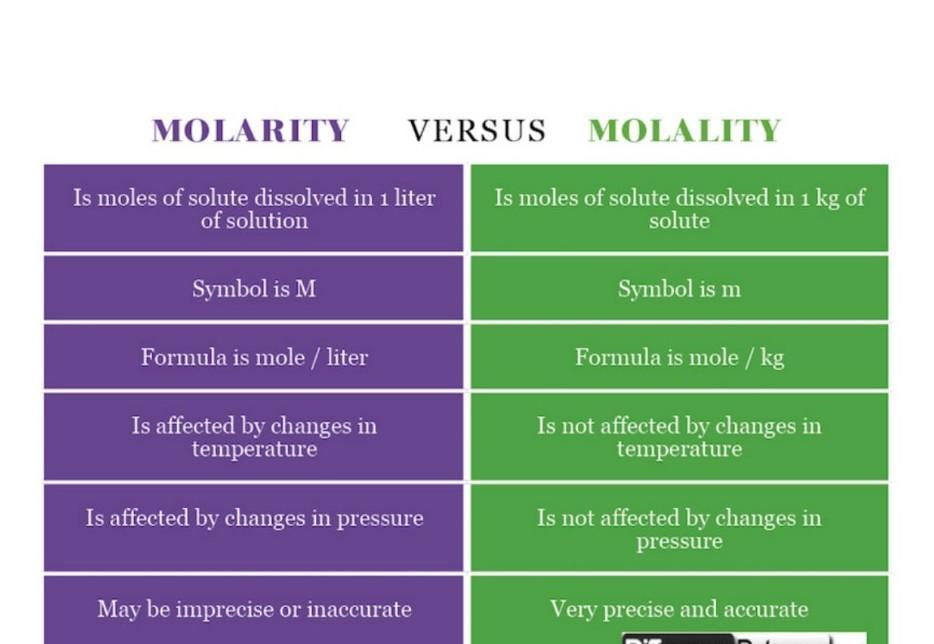
Molarity - Molality
The main difference between Molarity and Molality is that molarity focus on the number of mole of solute per litres of the given solution, while molality deals with the mole of solute per kg of the solvent. Molarity is represented by ‘M’ or Molar or mol/L, while Molality is represented by ‘m’ or Molal or mol/kg.
MOLARITY
Is moles of solute dissolved in 1 liter of solution
Symbol is M
MOLALITY
Is moles of solute dissolved in 1 kg of solute
Symbol is m
IF THIS HELPED YOU MARK ME BRAINLIST PLZ-
Author:
carsensih9
-
Rate an answer:
6
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years

