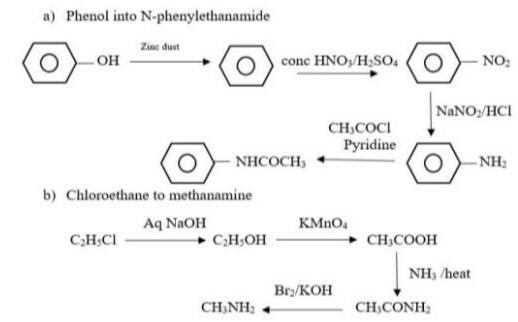
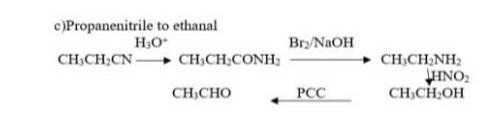
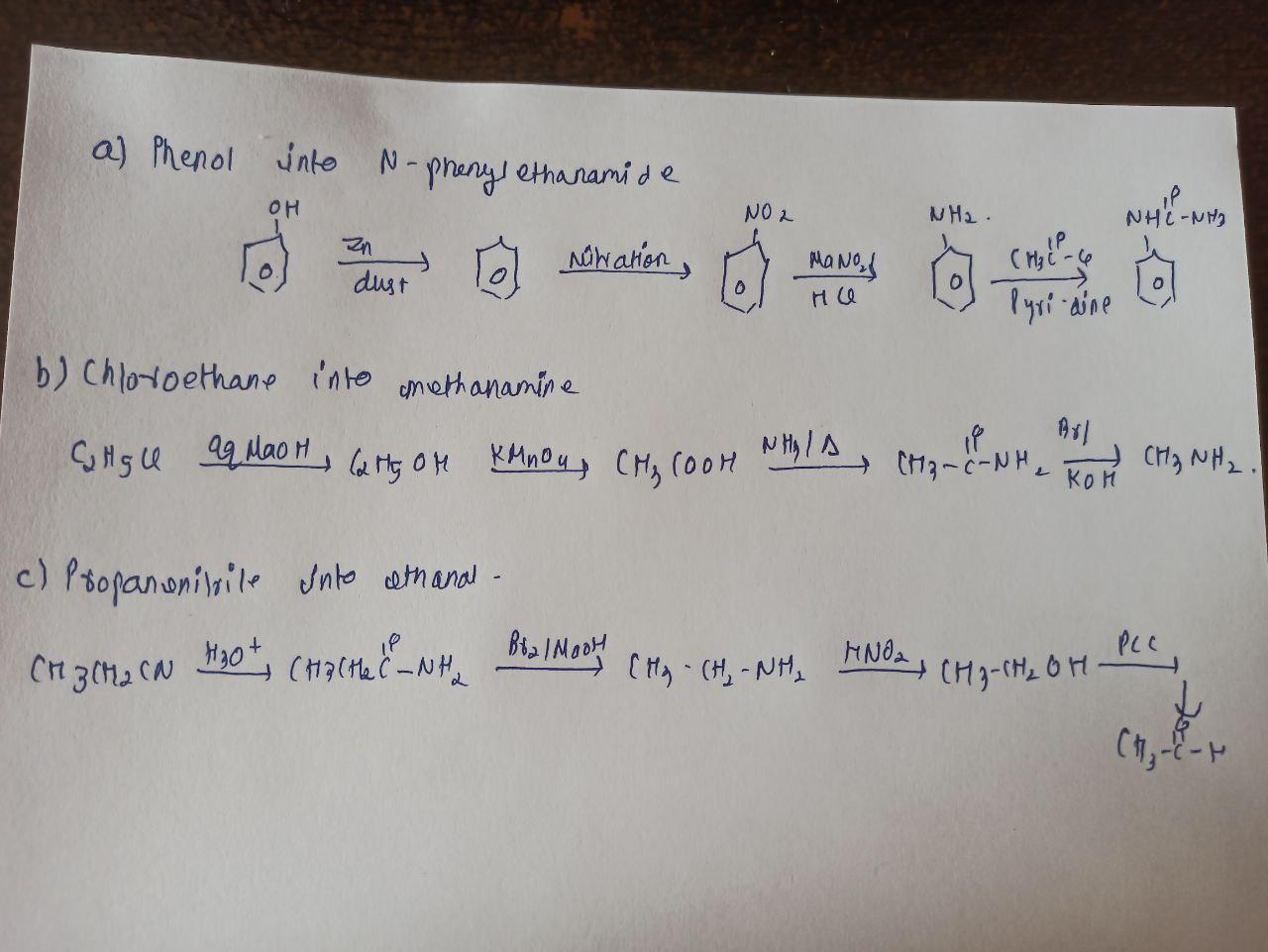
Which of the following sentences is punctuated correctly? O Ms. Giardi arrived before we did didn't she? O Ms. Giardi arrived, before we did, didn't she? O Ms. Giardi arrived before we did, didn't she? O Ms. Giardi arrived before we did. Didn't she?
Answers 2
Answer:
3 O Ms. Giardi arrived before we did, didn't she?
-
Author:
valerielpmu
-
Rate an answer:
9
Explanation:
ms giardi arrived before we did , didn't she?
-
Author:
persyfrederick
-
Rate an answer:
2
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years