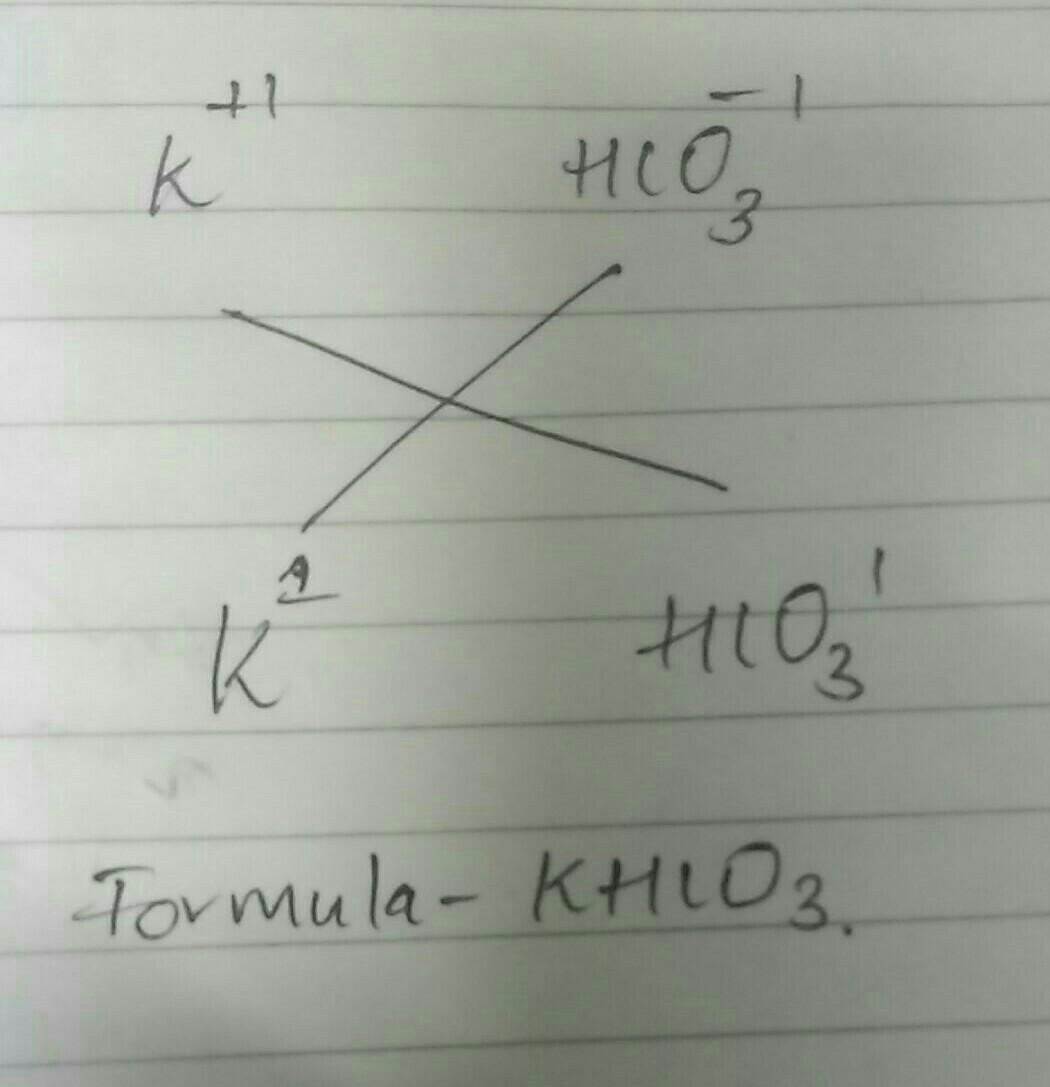
Answers 1
Answer:
The second one, while being the originally grammatically correct formulation, now sounds terribly old-fashioned
-
Author:
phillip777
-
Rate an answer:
2
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years