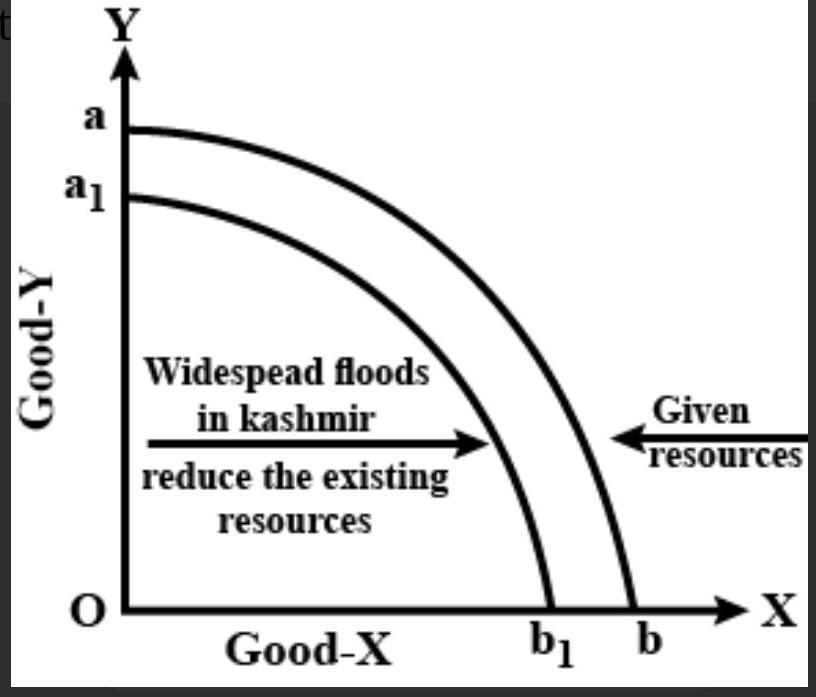
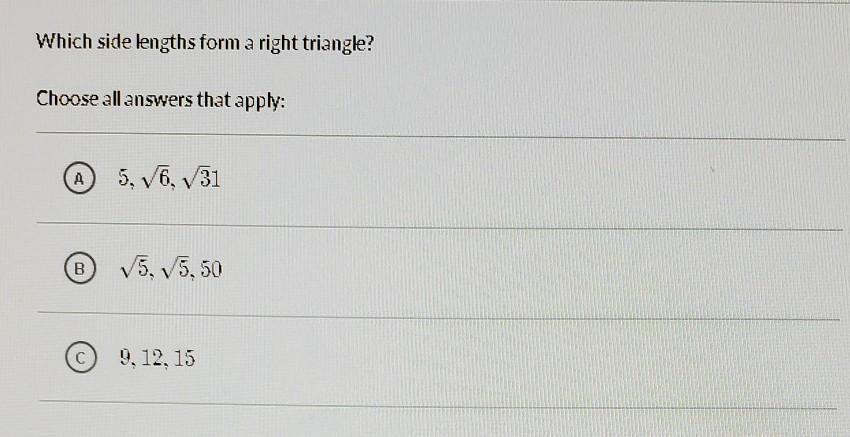
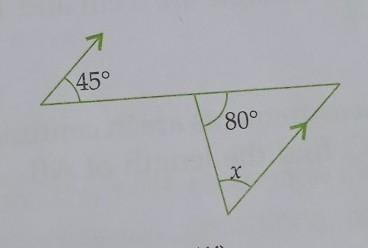
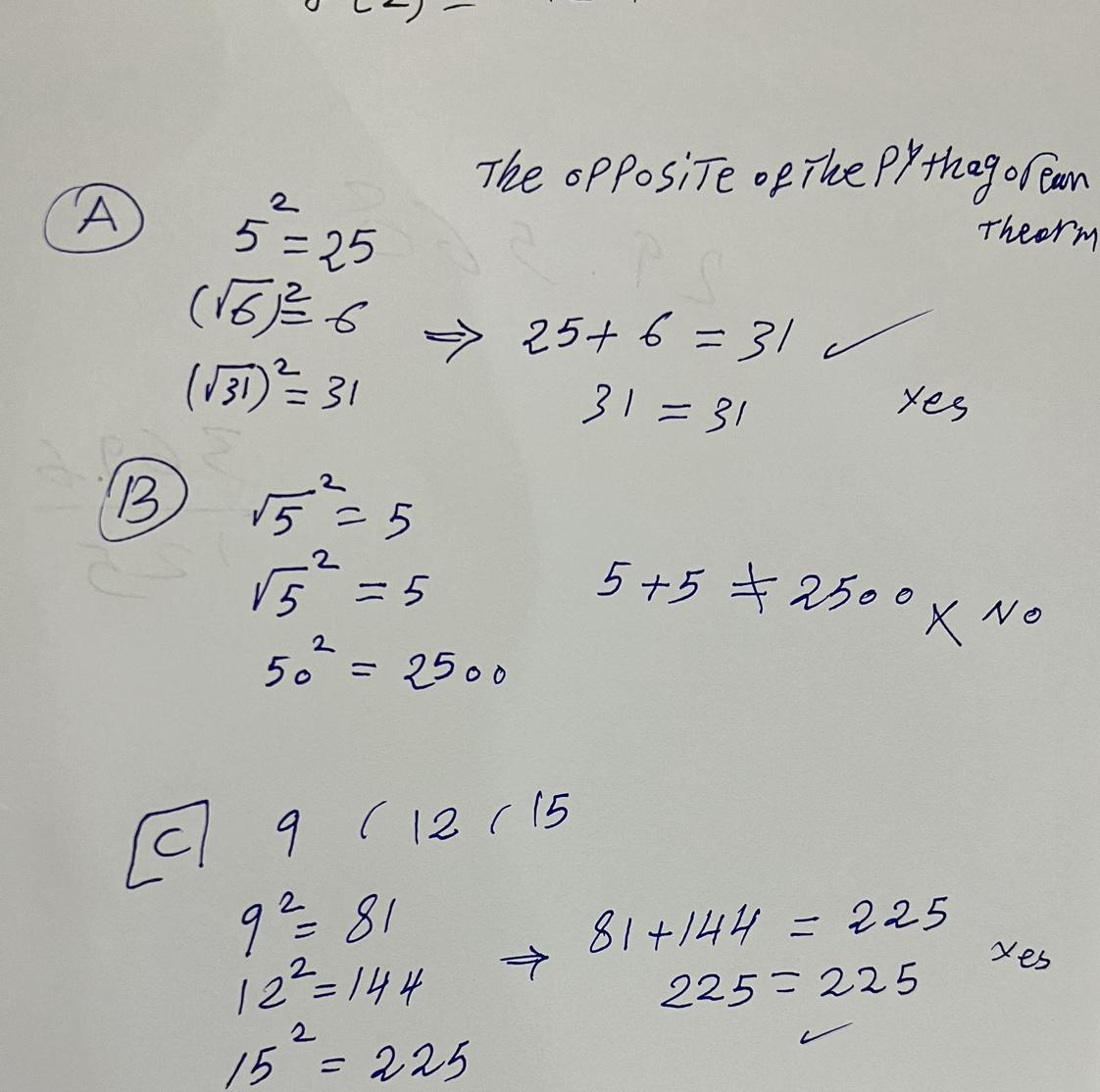NaCl in aqueous solution present in the form of ions surrounded by water molecules, which randomly move, but the main ionic attraction between Na and Cl is still exist loosely held, so that's why NaCl salt is also salt in water in disarranged form. my strong opinion is that it is a physical change not a chemical, am I right? many researcher still says it's a chemical change, create a confusion in mind, so need help?
-
Subject:
Chemistry -
Author:
haleyhenderson -
Created:
1 year ago
Answers 2
Answer:
NaCl is ionic salt and completely dissociate into ions when added into water and form aqueous solution of ions,
and we have,
NaCl → Na+ + Cl−
and ions get hydrated by the water molecules.
-
Author:
maddiesvkh
-
Rate an answer:
9
NaCl is ionic salt and completely dissociate into ions when added into water and form aqueous solution of ions,and we have,NaCl→Na + +Cl − and ions get hydrated by the water molecules.
-
Author:
shermankwhc
-
Rate an answer:
10
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years