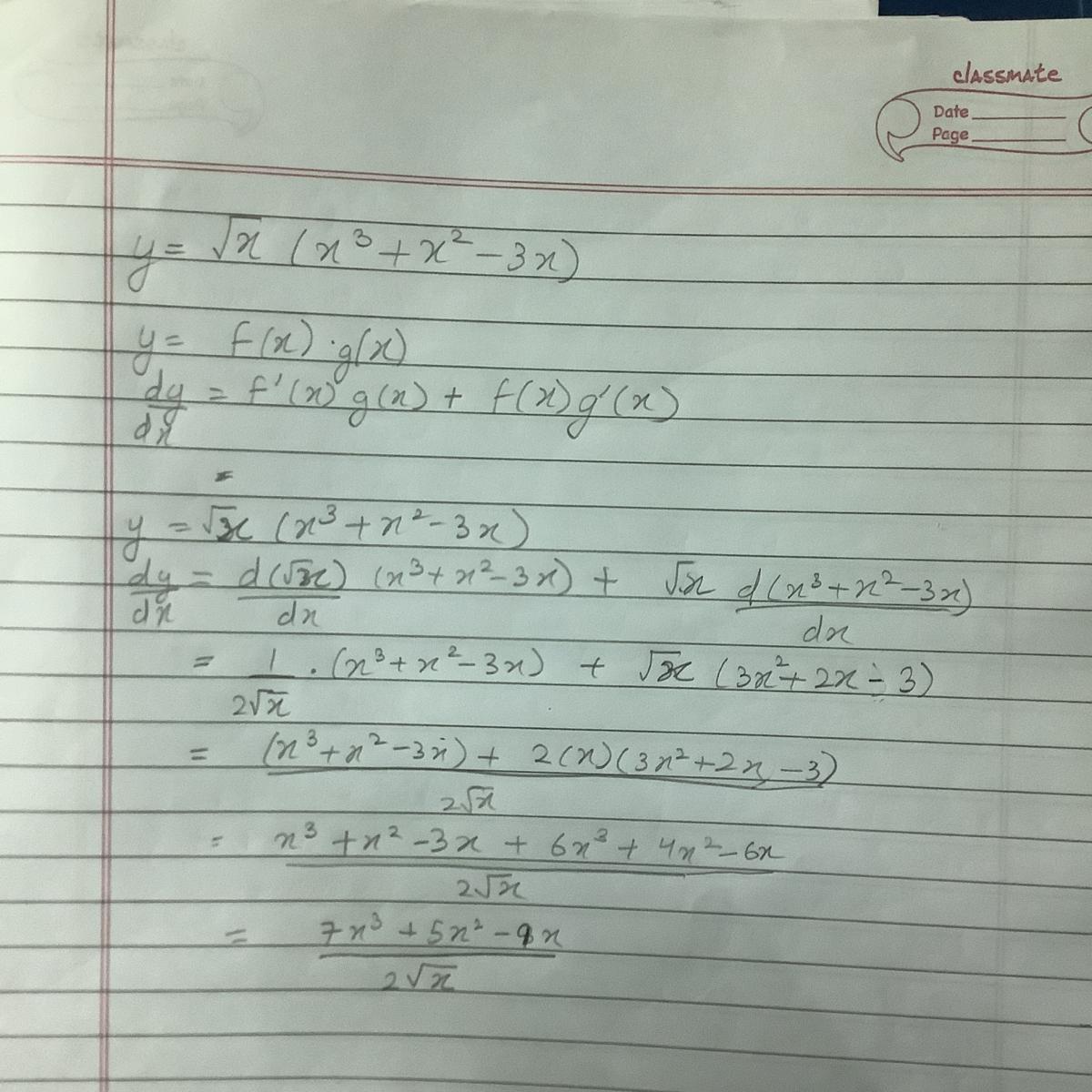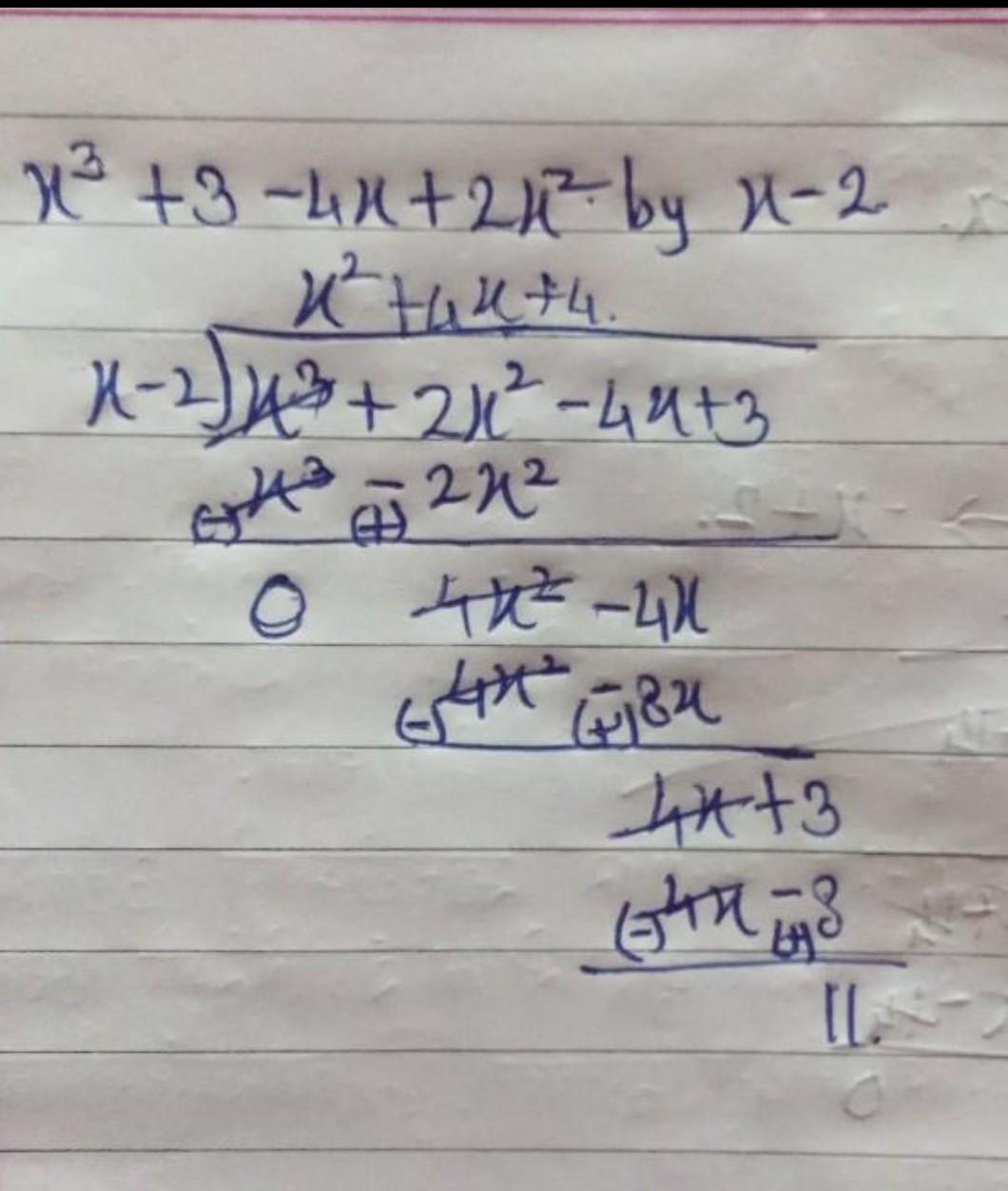If ‘x’ be the radius of first Bohr’s orbit of H-atom, the de-Broglie wavelength of an electron revolving in the second Bohr’s orbit will be 1) 6πx 2) 4πx 3) 2πx 4) 8πx
-
Subject:
Chemistry -
Author:
jaylangamble -
Created:
1 year ago
Answers 1
Explanation:
‘x’ be the radius of first Bohr’s orbit of
H-atom, the de-Broglie wavelength of an
electron revolving in the second Bohr’s orbit
will be
- 2πx
-
Author:
conorpdzq
-
Rate an answer:
7
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years