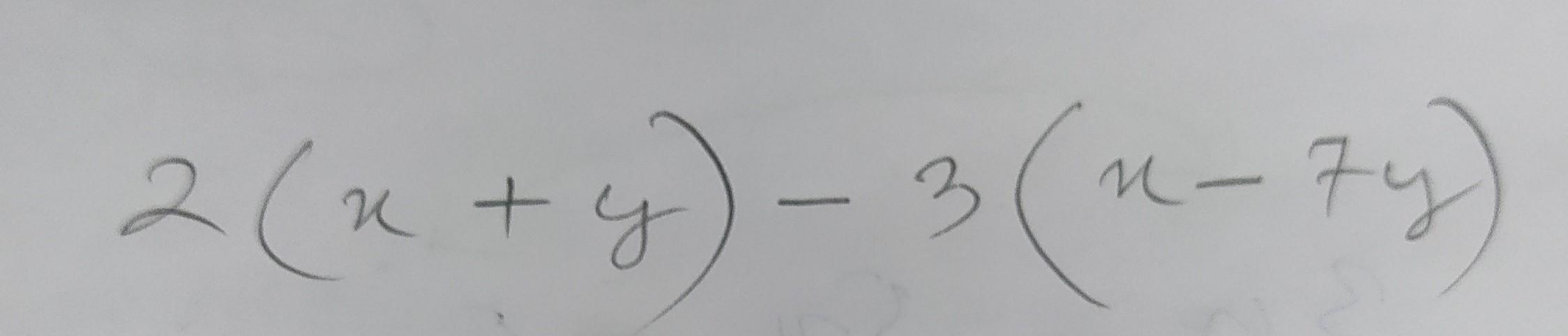Answers 2
Answer
my Pinterest id is Shafakat Umar
there will be pic of two girls pouting (≧∇≦)/
dekhna spelling id ka sahi hona chahiye
add now ok
-
Author:
emma14
-
Rate an answer:
13
Answer:
Explanation: Fe(s) +CuS[tex]O\x_{4}[/tex](aq)----> FeSO[tex]_{4}[/tex](aq) + Cu(s)
here Iron displaces or takes place of another element, a less reactive one here Copper in the product side to form Iron Sulphite. This is called displacement Reaction.
Displacement reaction only takes place when the element that would be displaced is less reactive that the displacing element according to the Reactivity Series
-
Author:
cindy lou who
-
Rate an answer:
6
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years