Answers 2
Answer:
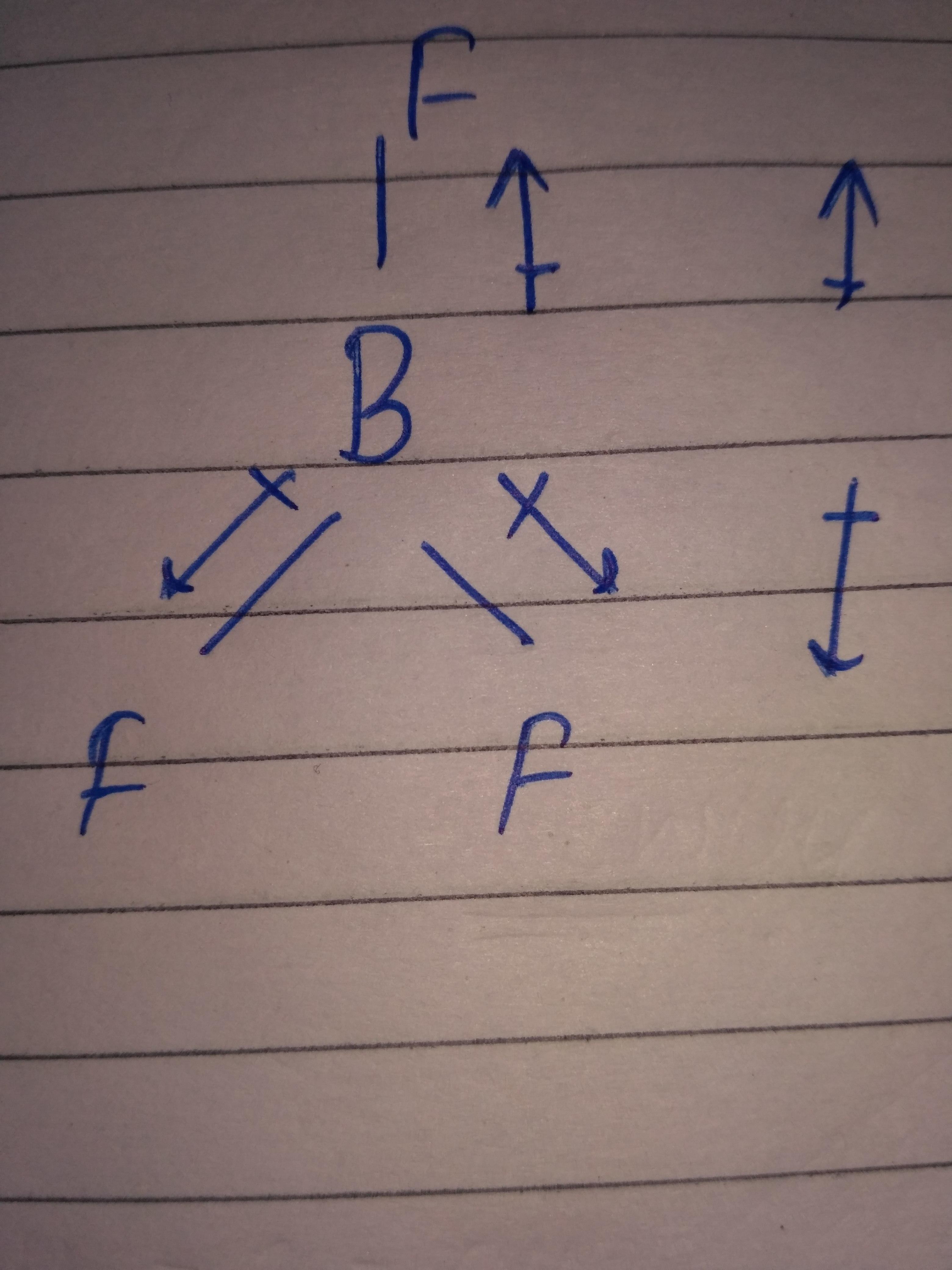
In BF3, the three flourine atoms are arranged in such a way that they form 120° each with the central boron atom(Trigonal planar geometry).Due to this symmetry, the individual B-F dipole moment cancels out resulting in the 0 dipole moment of BF3.-
Author:
pecansun9
-
Rate an answer:
6
Answer:
- Boron trifluoride is has zero dipole moment although it has three polar B—F bonds. This is because BF3 has sp2 hybridisation and regular trigonal planal geometry.
- And hence the individual dipole moments of polar bonds get cancelled and overall dipole moment is zero.
Explanation:
The three fluorine atoms are so attached to the boron that the resultant of two fluorine atoms cancel with the third one.
-
Author:
patrick685
-
Rate an answer:
4
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years