How many moles of nitrogen atoms are combined with 8.60 mol of oxygen atoms in dinitrogen pentoxide, N2O5hope it was helpful to u
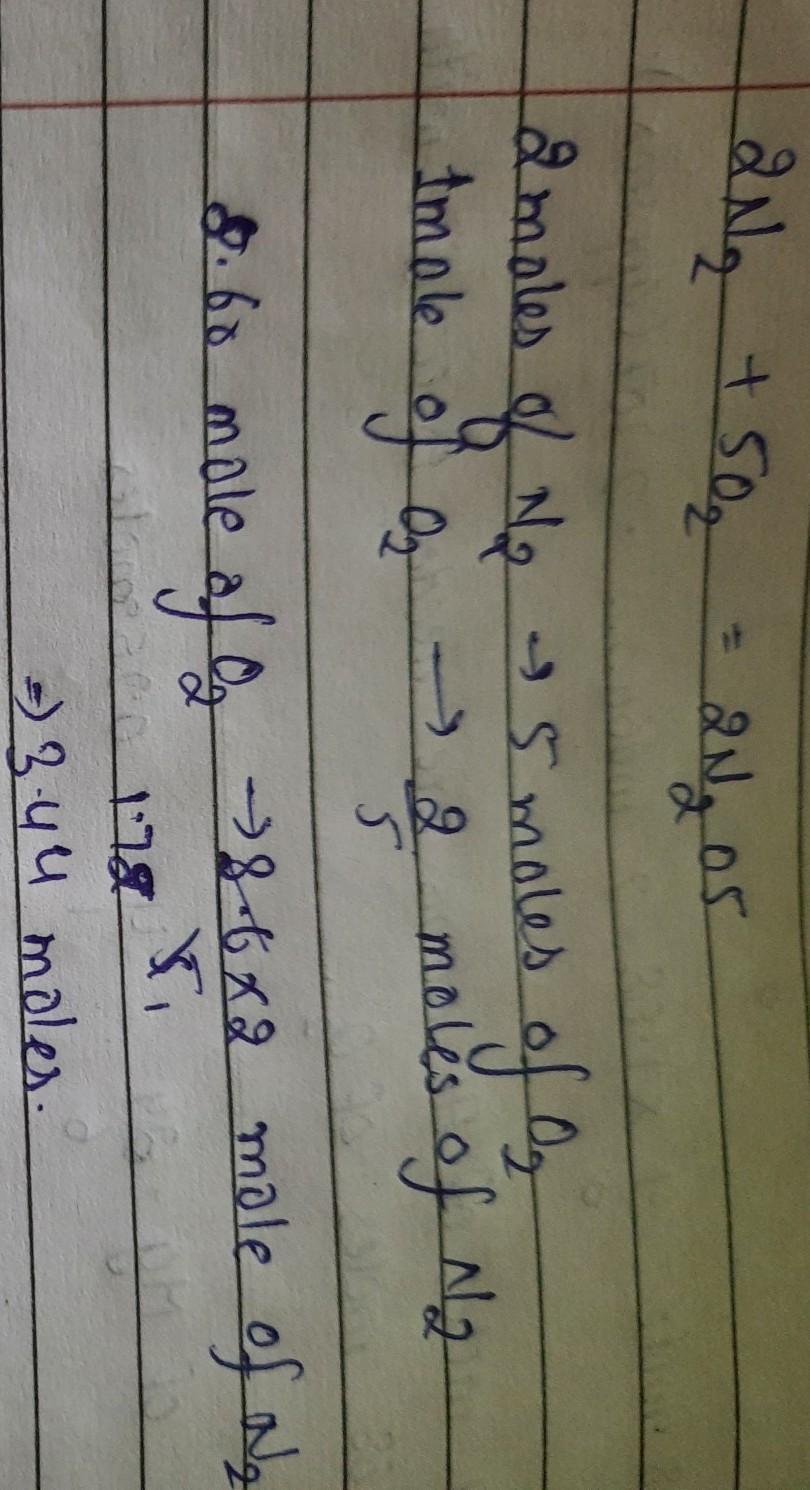
Answers 1
Given: 8.60 moles of Oxygen
To find: no. of moles of Nitrogen that can be combined with 8.6 moles of Oxygen.
Solution:The chemical formula of dinitrogen pantoxide is
N2O5.
Which means that for every 5 Oxygen atoms we require 2 atoms of Nitrogen.
So for 5 mol of O2 we require 2 mol of N2
therefore, 1 mol pf O2 require 2/5 mol of N2
Hence, 8.6 mol of O2 will require 2/5 × 8.6 mol of N2
which comes up to be 3.44 moles of N2.
So the no. of moles of Nitrogen that can be combined with 8.6 moles of Oxygen is 3.44
-
Author:
clickerbbum
-
Rate an answer:
9
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years
