Draw resonance structure showing +R effect aniline
Answers 1
backIconAnswer
NCERT Solutions
Popular Textbook Solutions
CBSE
ICSE
State Boards
Competitive Exams
Important Concepts
Other
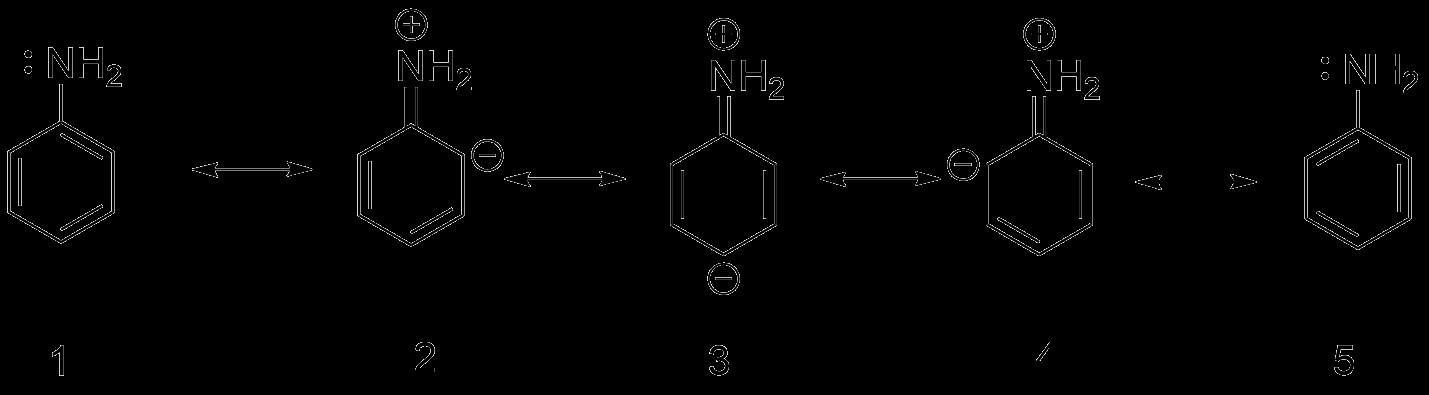
Draw the resonating structures of aniline.
VerifiedVerified
81k+ views
Hint:The resonance structures of aniline are drawn by first displacing the lone pair of electrons on the nitrogen to the bond between C and N. This results in formation of a double bond between C and N with N getting a positive charge due to the donation of electrons.
Complete answer:
- Aniline has the chemical formula C6H5NH2 . It is an organic compound that consists of a phenyl group attached to an amine group.
- Aniline is considered to be the simplest aromatic amine.
- Aniline has the odour of a rotten fish.
- Aniline has 5 resonating structures.
-
Author:
jaiden849
-
Rate an answer:
7