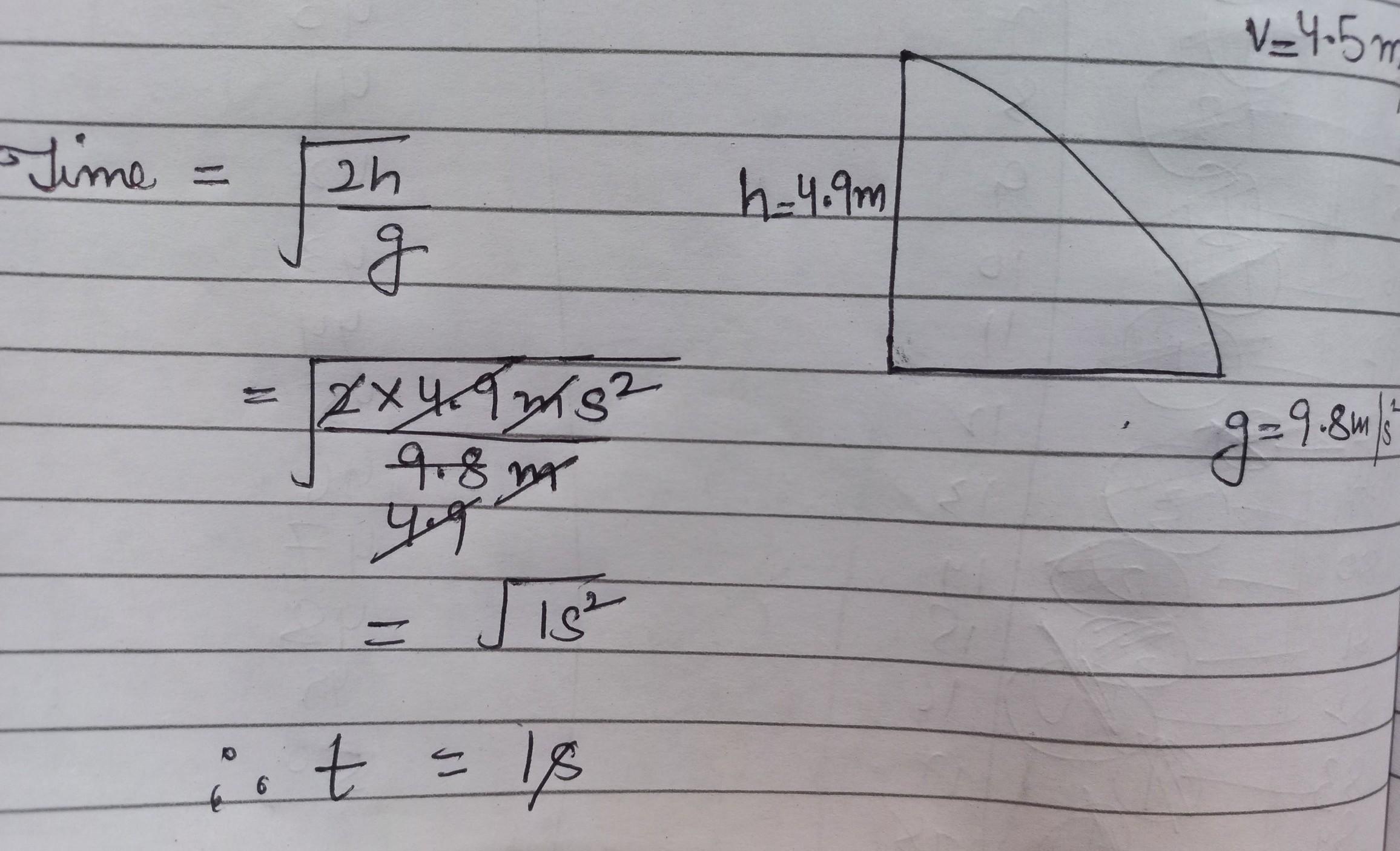
what is Grignard reagent ? State the action of water on methly magnesium bromide in dry ether with the help of chemical reaction.What is the action of Zn-HCL on ethyl iodide
Answers 1
Grignard's reagent is an organo-magnesium halide having the general chemical formula as (R-Mg-X) which is used to form a Carbon-Carbon bond and reduce an aldehyde or ketone to alcohol.
- "R" is the organic part of the compound. "X" is a halogen, otherly known as group 17 elements like Flourine, Chlorine, etc.
- The compound helps us to create the rather difficult C-C bond or carbon-carbon bond.
- In Grignard's reaction, the desired alkyl, allyl or any carbonyl group is added to the magnesium halide which is then reacted with an aldehyde or ketone to reduce it to alcohol and add the carbonyl group to the organic compound.
The reaction of water on methyl magnesium bromide in dry ether forms methane.
CH₃-Mg-Br + H₂0 --> CH₄ (methane) + Br-Mg-OH
The lone pair electrons on the oxygen atom attack the methyl carbon and remove the MgBr part from the compound and replace it with a Hydrogen atom to form methane.
The action of Zn-HCL on ethyl iodide is that it reduces ethyl iodide to ethane.
CH₃-CH₂-I + 2[H] [tex]\frac{Zn}{HCL}[/tex]--> CH₃-CH₃ (ethane) + HI
Zn/HCL acts as a reducing agent thus when reacted with ethyl iodide, the nascent Hydrogen atoms attack the iodine part of the compound and replace it thus forming ethane and Hydroflouride.
-
Author:
ninabuck
-
Rate an answer:
11