Ferric chloride solution is mixed to with aqueous sodium hydroxide
Answers 2
Answer:
Aqueous ferric chloride (FeCl3) reacts with aqueous sodium hydroxide (NaOH) to produce ferric hydroxide ( Fe(OH)3 ) and sodium chloride (NaCl). So, yellow-brown color solution will turn into a brown color precipitate.
Explanation:
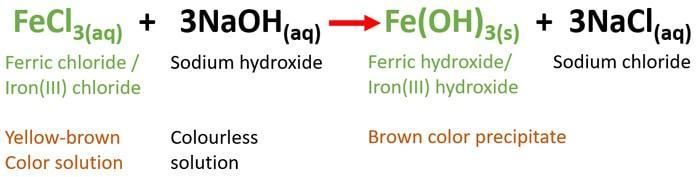
-
Author:
salomébjmg
-
Rate an answer:
20
Answer:
Aqueous ferric chloride (FeCl3) reacts with aqueous sodium hydroxide (NaOH) to produce ferric hydroxide ( Fe(OH)3 ) and sodium chloride (NaCl). So, yellow-brown color solution will turn into a brown color precipitate.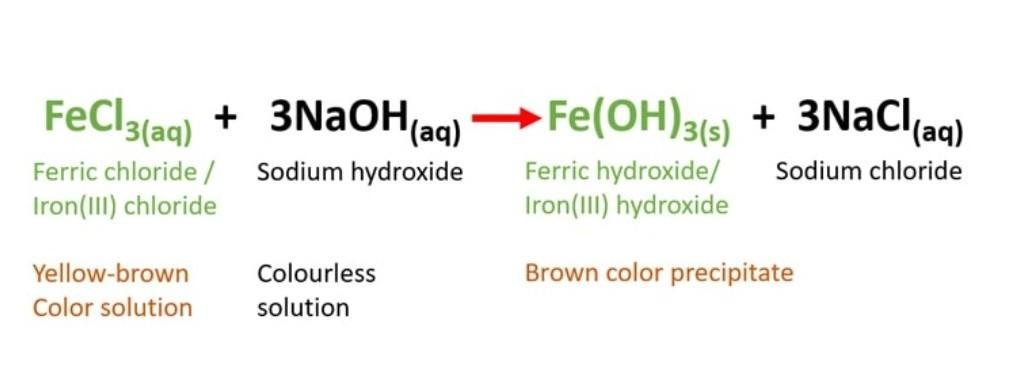
-
Author:
queeniecisneros
-
Rate an answer:
8
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years
