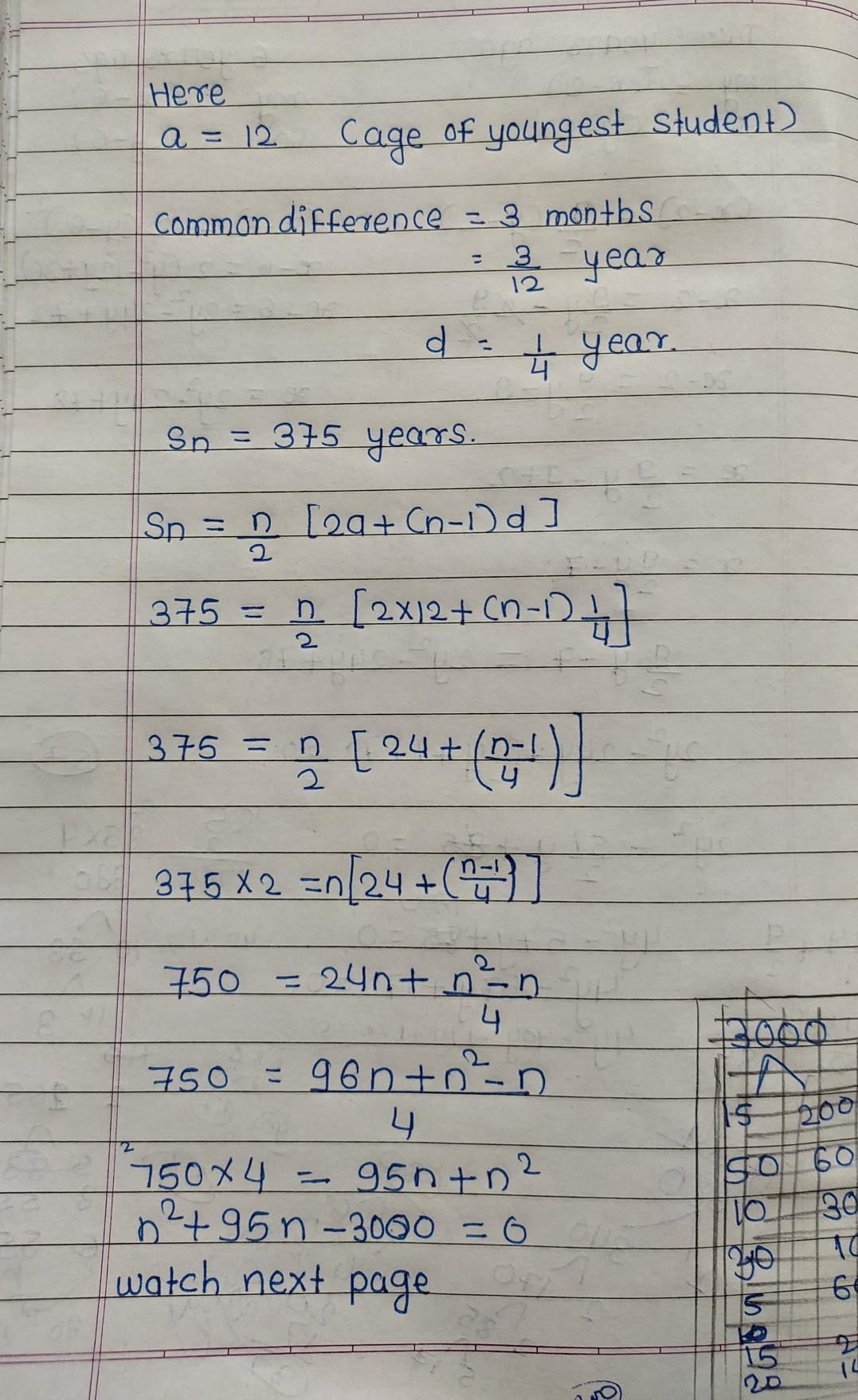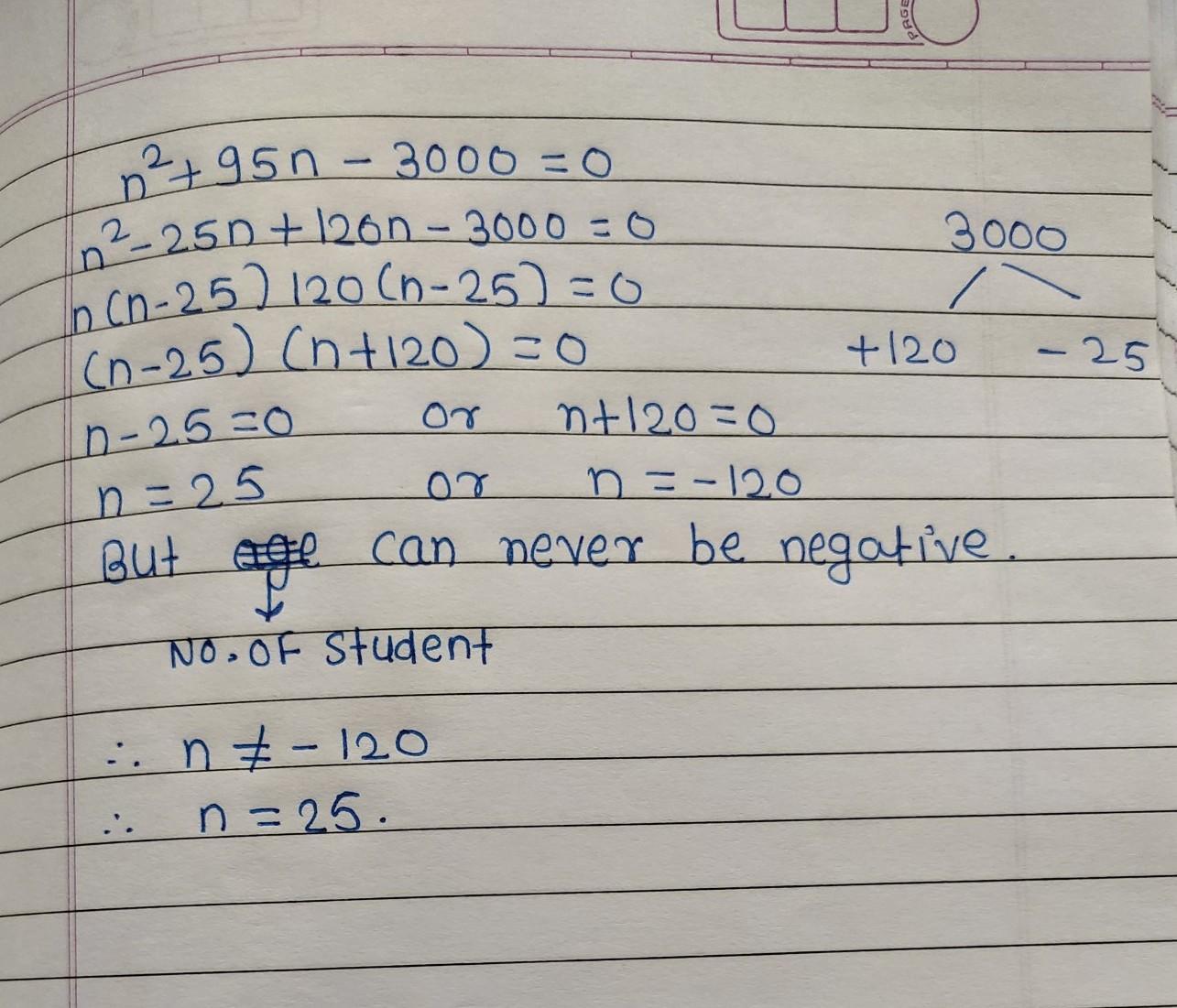Answers 2
Answer:
60.05 gm /mol
Explanation:
Molar mass ={2×12.011(C)+4×1.00794(H)+2×15.999(O)}.g.mol
−1
=60.05.g.mol
The molar mass of acetic acid is 60.05⋅g⋅mol
-
Author:
micahlevy
-
Rate an answer:
4
[tex]\star[/tex] Concept
_________________________________Molecular Mass is the sum of individual atoms in the molecule .Start the question by writing chemical formula of Acetic acid and the add the molar mass of individual atoms.
_________________________________Complete step by step answer :
[tex]\sf\implies[/tex] Chemical formula of Acetic acid [tex]\rightarrow[/tex] (C [tex]H_{3}[/tex] COOH)
[tex]\rightarrow[/tex] Atomic mass of C = 12
[tex]\rightarrow[/tex] Atomic mass of O = 16
[tex]\rightarrow[/tex] Atomic mass of H = 1
Molecular mass :
- Since , there are 2 carbon atoms
[tex]\rightarrow[/tex] 2 × atomic mass of C
- Since, there are 2 atoms of oxygen
[tex]\rightarrow[/tex] 2 × atomic mass of O
- Since, there are 4 atoms of hydrogen
[tex]\rightarrow[/tex] 4 × atomic mass of one hydrogen atom
[tex]\sf\implies[/tex] 2× 12 + 2 × 16 + 4 × 1
[tex]\sf\implies[/tex] 24 + 32 + 4
[tex]\sf\implies[/tex] 60
Since , Molecular mass is donated by u
[tex]\therefore[/tex] Molecular mass of Acetic acid is 60 u.
-
Author:
coryifax
-
Rate an answer:
9