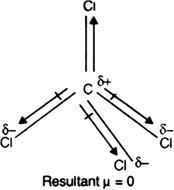
Explain why dipole moment of carbon tetrachloride is zero?
Answers 2
Answer:
In a carbon tetrachloride molecule the geometry of the structure of the molecule is tetrahedral. Chlorine is more electronegative with partial negative charge and carbon carries partial positive charge. ... Hence, the net dipole moment of the molecule is zero or it has no net dipole moment.
Explanation:
-
Author:
donovan677
-
Rate an answer:
5
The dipole moment of carbon tetrachloride is zero because of its tetrahedral geometry.
Explanation:
Carbon tetrachloride has [tex]sp^{3}[/tex] hybridization that is responsible for the tetrahedral geometry of the molecule. All the angles in a tetrahedral geometry are equal to 109.5°. Even though carbon and chlorine have an electronegativity difference the molecule has a net dipole moment of zero. This is due to the symmetric structure. Each bond has an individual dipole but it gets cancelled out in the molecule.
-
Author:
little bearmnxu
-
Rate an answer:
17