How many isomers are possible for the compound with the molecular formula C₄H₈? b) Draw the electron dot structure of branched chain isomer. c) How will you prove that C₄H₈ and C₅H₁₀ are homologues?
Answers 2
Answer:
(a) Four
(b) C4H8 and C5H10 are homologues as they differ in
● “- CH2-”
● differ in 14u molecular mass
● Same functional group
● Same general formula
Explanation:
MARK ME BRAINLIEST
-
Author:
harriettmqg
-
Rate an answer:
4
(a) Four isomers are possible for the compound with the molecular formula [tex]C_4H_8[/tex].
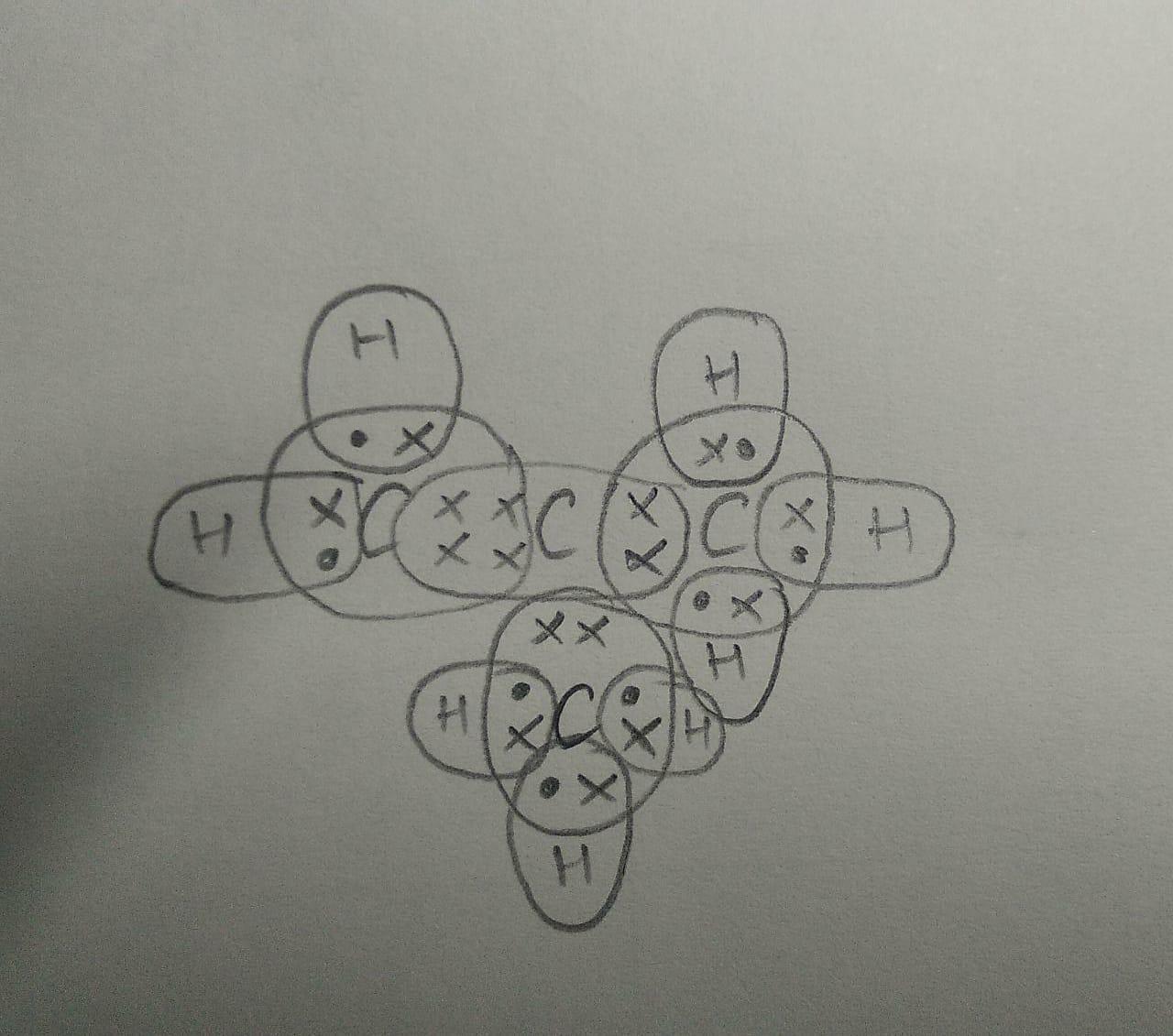
(b) The electron dot structure of the branched-chain isomer is attached below.
(c) We can say the compounds [tex]C_4H_8[/tex] , [tex]C_5H_{10}[/tex] are homologous by the following points:
1. Both the compounds have the same functional group.
2. Both the compounds have the same general formula.
3. Both the compounds have a difference of 14u in terms of molecular mass.
4. Bothe compounds differ by [tex]-CH_2[/tex].
- The four isomers are 1-butene, cis-2-butene, trans-2-butene, isobutylene.
- The electron dot structure is also known as the lewis dot structure which represents the valence electrons of an atom. The number of dots in the representation tells us the number of valence electrons.
- Homologous Series: It is a sequence of compounds with the same functional groups and chemical properties which differ by -[tex]CH_2[/tex].
-
Author:
mayhutchinson
-
Rate an answer:
9

