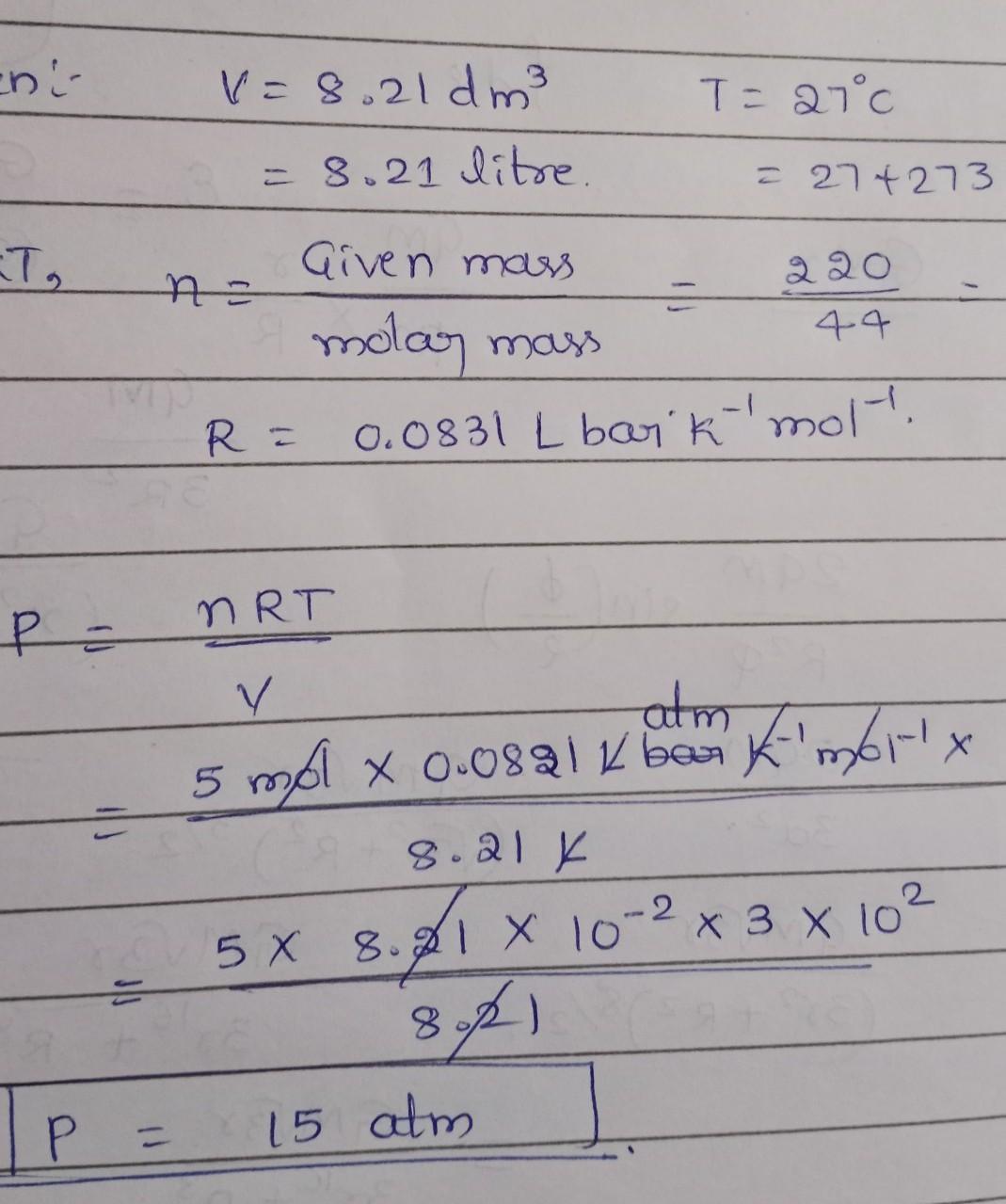Explanation:
Chapter 1
Chapter 2
Chapter 3
Chapter 4
Chapter 5
Chapter 6
Chapter 7
Chapter 8
Chapter 10
Kips IT/Cyber Beans Class 8 Chapter 9 Solutions APP DEVELOPMENT
A. Fill in the Blanks:
B. State True or False:
C. Application Based Questions:
D. Multiple Choice Questions:
E. Answer the following:
Other Chapter Solutions
Share this:
Related
Kips IT/Cyber Beans Class 8 Chapter 9 Solutions APP DEVELOPMENT
Brain Developer
A. Fill in the Blanks:
1. Hybrid Apps combine features of both Web apps and Native apps.
2. The abbreviation of the word ‘application’ is App.
3. Before installing an app on our device, the manufacturer of the app will ask for certain Permissions.
4. The Facebook app is an example of a Social Networking app.
5. The e-Commerce app that was founded by Sachin Bansal and Binny Bansal is Flipkart.
6. Desktop do not need web access and run independently on a computer.
7. We can pay utility bills using a Banking app.
B. State True or False:
1. Google Mapsis and commerce website. – FALSE
2. SDK stands for Software Design Kit. – FALSE
3. Hungama Music app brings your favorite tunes right to your device. – TRUE
4. Mobile apps are usually smaller in size as mobile devices have limited memory, – TRUE
5. Install SBI Buddy on your mobile device for the latest information on the global and Indian markets. – FALSE
C. Application Based Questions:
Sneha and her parents are planning to go for a movie. She wants to book the tickets online. Which app can she use to do the same?
BookMyShow App
Mannat had a school trip last month where she clicked various photographs. Now she wants to share these Photographs with her friends. Which app can she use?
WhatsApp
D. Multiple Choice Questions:
1. Reddit is a Social Networking site which is also called a News Aggregator app.
a) News Aggregator b) Banking c) Video Sharing
2. Appy Pie is an app which lets you build your own apps.
a) NCF App b) Reddit c) Appy Pie
3. Some app-builder apps let you build your own apps without the thorough knowledge of Programming Language.
a) OS b) Programming Language c) Brush
4. Hotstar is an Entertainment app.
a) Education b) Entertainment c) Social Networking
5. Buying or selling goods or services using electronic means is known as E-Commerce.
a) Money Control b) Finance c) E-Commerce
E. Answer the following:
How can you broadly categorise the apps? Explain any one category of apps.
Apps can be broadly as desktop apps. Web apps can be used on the desktop or a laptop. To run a desktop app, it must be first be installed on the desktop or the laptop. Examples are word processes, spreadsheet, point or Notepad.
What are the requirements for developing anapp by scratch?
To make an Android app, software such as Android software development kit is required, knowledge of Java is needed since most APIs use Java. You may also need to learn XML, HTML and CSS for designing an app.
What is the difference between Hybrid apps and Native apps?
1) Native apps – Mobile apps are usually developed for a specific operating system and device. These apps are developed keeping the device specifications in mind.
2) Hybrid apps – It contains the feature of both web apps and native apps. These apps require the device to be connected to the internet.
What is the use of the Mixer Brush Tool? How can you apply it to an image?
1) Desktop apps – These apps can be used on a desktop or a laptop. To run a desktop app, it must first be install on the desktop or the laptop.
2) Mobile apps – These apps run on a smartphones or a tablet. Some app may come preloaded on cell phones or tablets.
How can you download and install an app from the Google Play Store?
1) Tap on the Play Store icon.
2) Type the name of the app in the search bar.
3) It will display the relevant list of apps. select the most suitable one.
4) Tap the install button to install the app.
5) App will request you for permission to access.
6) Confirm your consent and the app will be downloaded.
Explain the importance of Educational Apps. Give an example to support your answer.
Educational apps are equally useful for both students and teachers. Students in the far-flung villages can easily access high quality educational resources, on the other hand teachers are able to distribute study material quickly, conduct test, and grade the students efficiently by using apps like Google classroom, Kahoot, etc.
With the help of suitable examples, explain the Photo editing tasks that can be done using Desktop, web, or Mobile Apps.
1) Desktop apps – Photoshop from Adobe is a photo editing software which must be installed on a desktop or laptop.
2) Web apps – Pixlr also provided photo editing software as web application available at https://www.pixlr.com/.
3) Mobile apps – Adobe Photoshop Express and Mobile Pixlr are photo editing apps for mobile devices which can be downloaded from Google Play Store or Apple App Store.