Write electronic configuration of Potassium (K) using orbital notation & orbital diagram method.
-
Subject:
Chemistry -
Author:
rodolfooneal -
Created:
1 year ago
Answers 2
Answer:
MARK as Brainlist if it helps you..
thank you !
-
Author:
ralphie6nbx
-
Rate an answer:
10
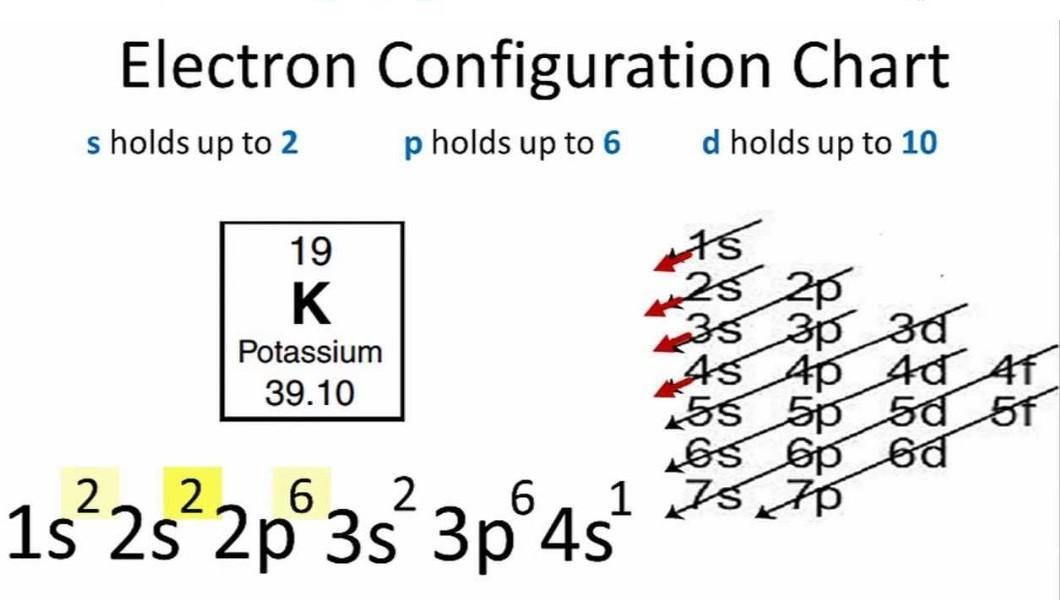
Potassium's atomic number is 19. This means that every atom of potassium has 19 protons in its nucleus.
In a neutral atom, the number of protons is equal to the number of electrons.
So the electron configuration of potassium will involve 19 electrons.
The full electron configuration of potassium is 1s2 2s2 2p6 3s2 3p6 4s1
The noble gas notation is [Ar]4s1
-
Author:
shnookienz1z
-
Rate an answer:
19
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years
