In H - atom, electron transits from 6^th orbit to 2^nd orbit in multi step, then total spectral lines (without Balmer series) will be: a.6 b.10 c.4 d.0
Answers 1
Answer:
total spectral lines is 10
b is the correct answer
Explanation:
the circle i have drawn are the no of total spectral lines .....
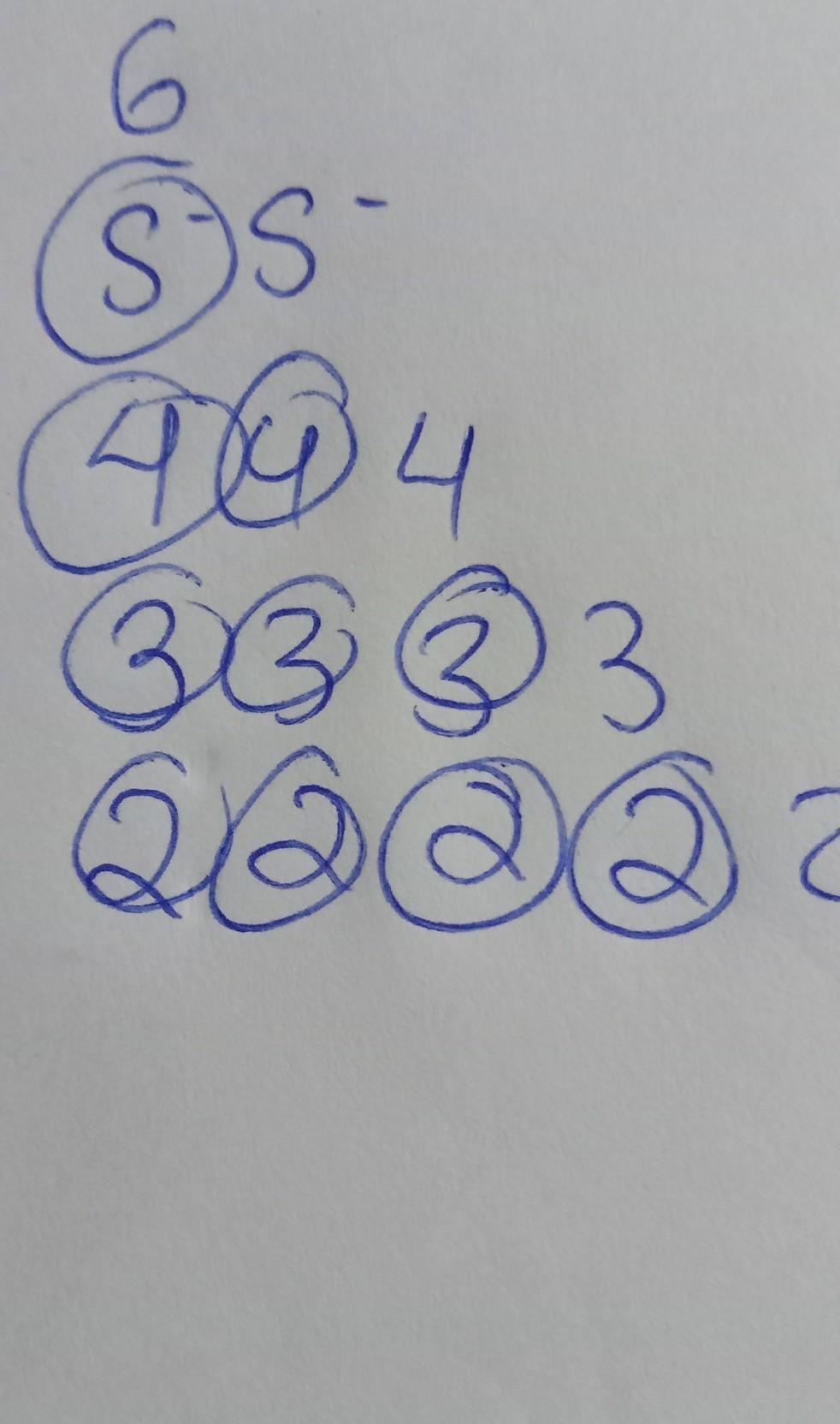
-
Author:
gisselleqz7p
-
Rate an answer:
8
If you know the answer add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years

